1568
Evaluation of the Glymphatic System Using the DTI-ALPS Index in Patients with Glioma1Department of Radiology, Affiliated Hospital of Xuzhou Medical University, Xuzhou, China, 2Philips Healthcare, Shanghai, China
Synopsis
Keywords: Tumors, Diffusion Tensor Imaging, edema
We evaluated the function of the human glymphatic system (GS) in patients with glioma using diffusion tensor imaging analysis along with the perivascular space (DTI-ALPS). Thirty-seven patients with glioma and 34 healthy controls (HCs) were recruited for analysis. We found that DTI-ALPS index on the ipsilateral lesion side was significantly decreased, but not on the contralateral side in glioma when compared with HCs. The decreased DTI-ALPS index was significantly correlated with the volume of peritumoral edema (PTBE), but not the volume of tumor. This study suggested the PTBE may be an independent factor that affects the GS in glioma patients.Introduction
The most common complication of glioma is the increase of intracranial pressure caused by tumor and peritumor edema (PTBE), which will further lead to the decrease of cerebral blood flow, thus causing a vicious circle of cerebral ischemia, hypoxia and edema [1]. Recent studies on glioma animal models further suggest that the normal outflow pathway of cerebrospinal fluid in cranial cavity is blocked, resulting in the disruption of the dynamic balance between cerebrospinal fluid (CSF) and interstitial fluid (ISF) [2].The glymphatic system (GS) was first discovered by Iliff et al. [3] through two-photon imaging of small fluorescent tracers in 2012. Previous studies have found that brain disorders can affect the GS, including brain oedema, blood–brain barrier (BBB) disruption, immune cell infiltration, neuroinflammation, and neuronal apoptosis [4]. As suggested by the above studies, diffusion tensor imaging analysis along with the (perivascular space, PVS) (DTI-ALPS) can be used as a quantitative imaging biomarker to monitor the ability of the GS [5]. Thus, the aim of this study was to investigate the pathological features of GS in patients with glioma using this new method of DTI analysis.Materials and Methods
Subjects: We recruited a convenience sample of 37 hospitalized patients with glioma. All patients received conservative treatment, and magnetic resonance imaging (MRI) was performed on each patient one month later.MRI Data Acquisition: All subjects underwent 3.0 Tesla MRI scanning (Elition, Philips Healthcare, Best, the Netherlands) with a 32-channel head coil. High-resolution T1-weighted images (T1WI) were acquired by using a three-dimensional (3D) gradient echo sequence, which provided isotropic voxels of 1 mm × 1 mm × 1 mm, repetition time (TR), 6.7 ms; echo time (TE), 3.0 ms; field of view (FOV), 256 mm × 256 mm; number of slices, 192; and flip angle, 8°. DTI data were obtained using the following parameters: TR/TE = 4825/77 ms, number of diffusion gradient directions = 64, b value =1000 s/mm2 and providing voxels of 2 mm × 2 mm × 2 mm.
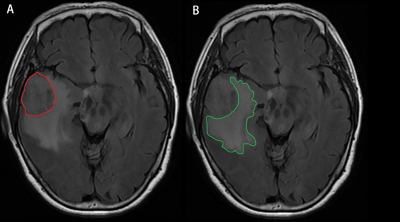
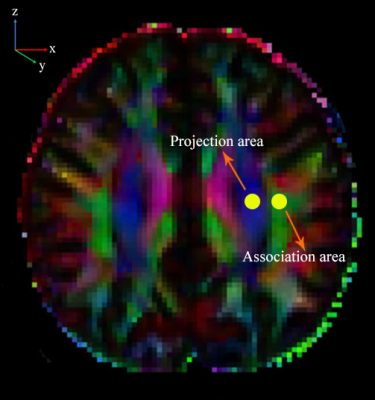
MRI Data Processing: The tumor and PTBE were delineated manually by two radiologists on individual high-resolution T1WI images using ITK-SNAP (http://www.itksnap.org) (Figure 1), and the volume was automatically generated. Each individual volume obtained from each radiologist was averaged as the final result.DTI Studio software was used in this study to measure DTI metrics (https://www.mristudio.org/). Briefly, the steps were as follows: (i) raw DTI data of a single individual were imported into the software; (ii) automatic image registration was performed; and (iii) the diffusion tensor was calculated, including a colour-coded fractional anisotropy (FA) map and diffusivity in the directions of the x-axis, y-axis, and z-axis (Figure 2). We evaluated the diffusivity along the direction of the PVS to further calculate the DTI-ALPS index [5-7]. DTI-ALPS index = mean (Dxxproj, Dxxassoc)/mean (Dyyproj, Dzzassoc).
Statistical Analysis: Intraclass correlation coefficient (ICC) statistics were used to assess interobserver agreement for DTI parameter measurements and the delineation of ipsilateral lesion volume. A chi-square test and a two-sample t test was used to observe sex and age differences between participants in the glioma and HC groups, respectively. Differences in the DTI-ALPS index among the ipsilateral lesion side/the contralateral side of glioma patients and HCs were tested using one-way ANOVA tests. Pearson correlation analyses were carried out to test whether DTI-ALPS data of patients with glioma significantly correlated with the volume of tumor or PTBE.
Results
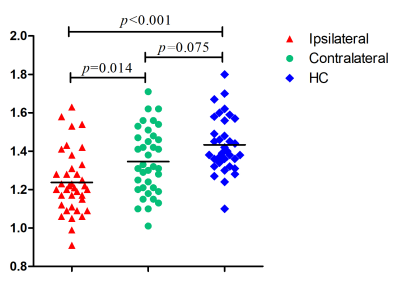
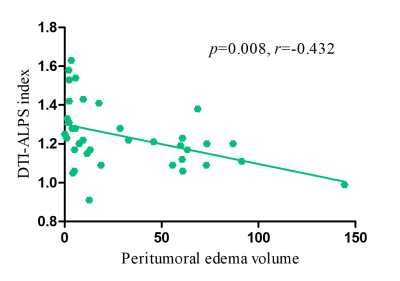
There was no difference between groups in age and sex. One-way ANOVA tests showed that there were significant differences in DTI-ALPS among the three groups (p < 0.001, F = 13.175). The DTI-ALPS index of the patient on the ipsilateral lesion side (1.23 ± 0.16) was significantly lower than that on the contralateral side (1.34 ± 0.17) (p = 0.014), as well as lower than that of the HCs. the (1.43 ± 0.15) (p < 0.001). However, no significant differences were found in the DTI-ALPS index on the contralateral side between patients and HCs (p = 0.075) (Figure 3). The DTI-ALPS index of the ipsilateral lesion side was significantly correlated with disease duration (p = 0.008, r = -0.432) (Figure 4).Discussion
The main goal of the current study was to determine the pathological changes in the GS in patients with glioma by means of DTI analysis. The lower DTI-APLS index on the ipsilateral lesion side indicated the GS disruption. Interestingly, we found no significant difference in DTI-ALPS on the contralateral side between participants in the glioma and HC groups, it indicated that GS may be an independent system in bilateral cerebral hemispheres, which is similar to our previous research[8]. The possible reason is that blood vessels are one of the most important parts of the GS. In addition, we found that PTBE is the main factor leading to the decrease of GS, which suggested that PTBE is the main cause of CSF-ISF exchange disorder, which is consistent with previous studies[9]. Overall, these findings have significant implications for the understanding of PTBE of glioma from a new perspective.Acknowledgements
The authors thank the patients who participated in this study and the staff of the Department of Radiology.References
[1] J. Y. Fan, C. Kirkness, P. Vicini, R. Burr and P. Mitchell, “Intracranial pressure waveform morphology and intracranial adaptive capacity,” Am J Crit Care, vol. 17, no. 6, pp. 545-554, 2008.
[2] Q. Ma, F. Schlegel, S. B. Bachmann et al., “Lymphatic outflow of cerebrospinal fluid is reduced in glioma,” Sci Rep, vol. 9, no. 1, pp. 14815, 2019.
[3] J. J. Iliff, M. Wang, Y. Liao et al., “A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β,” Science Translational Medicine, vol. 4, no. 147, pp. 147ra111, 2012.
[4] T. Lv, B. Zhao, Q. Hu and X. Zhang, “The glymphatic system: a novel therapeutic target for stroke treatment,” Frontiers in Aging Neuroscience, vol. 13, pp. 689098, 2021.
[5] T. Taoka, Y. Masutani, H. Kawai et al., “Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer's disease cases,” Japanese Journal of Radiology, vol. 35, no. 4, pp. 172–178, 2017.
[6] G. Yang, N. Deng, Y. Liu, Y. Gu and X. Yao, “Evaluation of glymphatic system using diffusion MR technique in T2DM cases,” Frontiers in Human Neuroscience, vol. 14, pp. 300, 2020.
[7] X. Ma, S. Li, C. Li et al., “Diffusion tensor imaging along the perivascular space index in different stages of Parkinson's disease,” Frontiers in Aging Neuroscience, vol. 13, pp. 773951, 2021.
[8] C. Zhang, J. Sha, L. Cai et al., “Evaluation of the Glymphatic System Using the DTI-ALPS Index in Patients with Spontaneous Intracerebral Haemorrhage,” Oxid Med Cell Longev, vol. 2022, pp. 2694316, 2022.
[9] C. H. Toh and T. Y. Siow, “Factors Associated With Dysfunction of Glymphatic System in Patients With Glioma,” Front Oncol, vol. 11, pp. 744318, 2021.
Figures