1557
High Resolution Spinal Cord Imaging at 7T with Rosette Trajectory and Compressed Sensing.1Department of Electrical and Computer Engineering, Auburn University, Auburn, AL, United States, 2Auburn University MRI Research Center, Auburn University, Auburn, AL, United States
Synopsis
Keywords: Spinal Cord, New Trajectories & Spatial Encoding Methods, Rosette Imaging, Compressed Sensing.
MRI is very useful to investigate spinal cord pathologies non-invasively. However, signal to noise ratio (SNR), spatial resolution and motion artifacts are some of the main challenges in spinal cord MRI. Ultra high field MRI such as 7T, can improve the SNR enabling high spatial resolution. The inherent property of low susceptibility to motion of non-Cartesian imaging technique such as Rosette, can improve the motion related artifacts. So the goal of this study was to develop a technique for high-resolution spinal cord imaging at 7T using Rosette MRI.Introduction
MRI is a valuable tool and routinely used for diagnosing spinal cord pathologies such as tumors, traumas, neurodegenerative diseases and vascular malformations. The efficiency to detect subtle pathological features in the spinal cord by MRI is limited by the signal to noise ratio (SNR), motion artifacts and spatial resolution 1, 2. Ultra high magnetic field such as 7T can potentially improve the diagnosis using increased SNR and improved susceptibility contrast to support high spatial resolution. The artifacts introduced to the images due to breathing motion in routine Cartesian MRI can be improved by using the non-Cartesian imaging techniques such as Spiral, Rosette imaging etc. In spite of the potential advantage of the non-Cartesian acquisition techniques, it has not been explored for the spinal cord imaging thoroughly. A recent study investigated Spiral imaging on the spine at 1.5T 3, which demonstrated the advantage of non-Cartesian MRI of the spinal cord. The advantage of Rosette imaging is the ability of flexible trajectory design, self-correction of inhomogeneity and motion insensitivity 4-6. In this study, we demonstrate high-resolution MRI of the spinal cord using Rosette trajectory at 7T. We also demonstrate the feasibility of compressed sensing for under-sampled Rosette acquisition to speed up the scan time.Methods
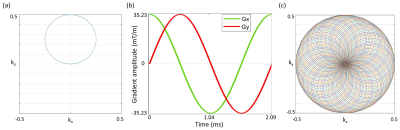
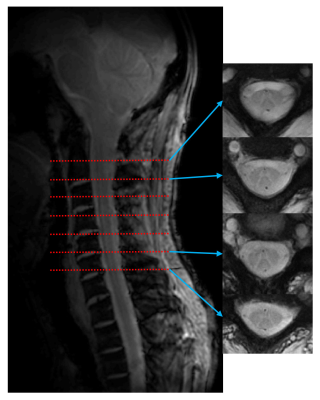
The Rosette trajectories oscillate in the radial direction about the origin of k-space with angular frequency $$$ω_{1}=2πf_{1}$$$, simultaneously rotating in the kx-ky plane with angular oscillation frequency $$$ω_{2}=2πf_{2}$$$. The k-space trajectory is given by 4-6 $$k(t)=k_{max}sin(ω_{1}t)e^{iω_{2}t}$$ Where $$$k_{max}=\frac{N_{x}}{2.FOV}$$$ is the highest spatial frequency, Nx is the matrix size and FOV is the field of view. The value of f1 and f2 can be chosen based on the intended shape of the k-space trajectory. We chose f1=f2, which results in a circular trajectory for a single shot 5 (Fig 1a). Corresponding gradient was calculated using $$$G(t)=\frac{2π}{γ} \frac{dk(t)}{dt}$$$, where γ=1H gyromagnetic ratio. Gradients along X and Y axes for the Rosette trajectory are shown in Fig 1b. Total number of shots to fill up the k-space based on the intended image resolution was $$$N_{sh}=π\frac{N_{x}}{2}$$$ 5 (Fig 1c).All the experiments were performed using Siemens 7T Magnetom with 8 channel spine coil. The peak gradient and slew rate of the scanner were 70 mT/m and 200 mT/m/ms respectively. Two subjects participated the study. Rosette imaging parameters were: FOV=192 mm, matrix=384, in-plane resolution=0.5×0.5 mm, slice thickness=4mm, 7 slices, flip angle=390, f1=f2=1500 Hz, number of shots=603, TR=300 ms. For Rosette imaging, multi-echo images were acquired at TE=3, 7.8 and 15 ms. To compare the Rosette images, single echo FLASH images were also acquired with same FOV, matrix, in-plane resolution, slice thickness and number of slices. Other parameters for the FLASH acquisition were: TE=3 ms, TR=40 ms and flip angle=100.
Rosette images were reconstructed for each slice using 2D gridding on a two-fold oversampled grid with a Kaiser-Bessel kernel window W=4 7 and density compensation was applied 8. Images were reconstructed from 603 shots and reduced number of shots. Compressed sensing 9 was applied to reconstruct the Rosette images from 201 and 100 shots of the original 603 shots.
Results
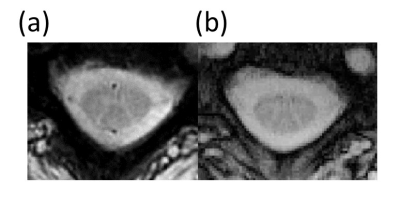
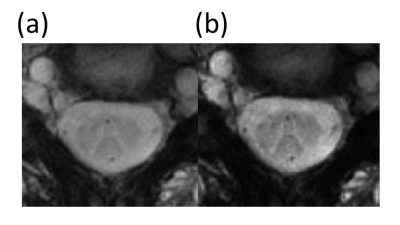
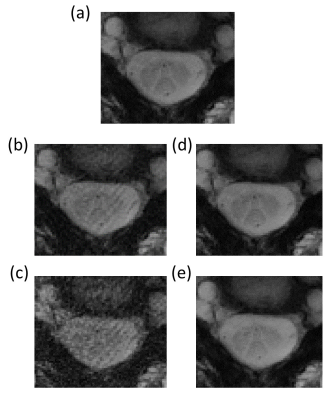
Maximum gradient amplitude was 35.23 mT/m and maximum slew rate was 105.7 mT/m/ms for the chosen Rosette design. Representative Rosette images (603 shots) from 4 slices on the spine at TE=3 ms are shown in Fig 2. Reduced blurring and sharper contrast is visible in Rosette images when compared with FLASH images at same echo time (TE=3 ms) (Fig 3). Averaging multiple echo time images (TE=3, 7.8 and 15 ms) significantly improves the contrast between gray and white matter in the spinal cord (Fig 4)10. Compressed sensing reconstruction of under-sampled data (100 and 201 shots vs 603 shots) does not show reduction in image quality (Fig. 5). Under-sampled Rosette acquisition with compressed sensing reconstruction allows us to reduce the acquisition time by up to 6x and potentially help with patient comfort and reduced motion blurring.Discussion
This study is the first report to demonstrate high-resolution spinal cord Rosette MRI with the application of compressed sensing at 7T. Our result shows that high-resolution spine imaging is feasible using Rosette imaging with improved contrast. As Rosette is insensitive to bulk motion, this technique can also reduce the motion artifacts compared to the routine Cartesian imaging. Application of compressed sensing can reduce the sampled data size and decrease the total acquisition time. Rosette imaging is ideally suited for magnetization prepared contrast imaging; therefore, it can be very useful in clinical applications for spinal cord pathology diagnosis.Acknowledgements
No acknowledgement found.References
1. Gilmore CP, Geurts JJ, Evangelou N, Bot JC, van Schijndel RA, Pouwels PJ, Barkhof F, Bo L. Spinal cord grey matter lesions in multiple sclerosis detected by post-mortem high field MR imaging. Mult Scler. 2009;15(2):180-188.
2. Bo L, Geurts JJ, Ravid R, Barkhof F. Magnetic resonance imaging as a tool to examine the neuropathology of multiple sclerosis. Neuropathol Appl Neurobiol. 2004;30(2):106-117.
3. Robison RK, Cornejo P, Kuwabara M, Ooi MB, Temkit H, Miller JH. Evaluation of axial gradient Echo spiral MRI of the spine at 1.5 T. Magn Reson Imaging. 2022;89:24-32.
4. Noll DC. Multishot rosette trajectories for spectrally selective MR imaging. IEEE Trans Med Imaging. 1997;16(4):372-377.
5. Schirda CV, Zhao T, Andronesi OC, Lee Y, Pan JW, Mountz JM, Hetherington HP, Boada FE. In vivo brain rosette spectroscopic imaging (RSI) with LASER excitation, constant gradient strength readout, and automated LCModel quantification for all voxels. Magn Reson Med. 2016;76(2):380-390.
6. Schirda CV, Tanase C, Boada FE. Rosette spectroscopic imaging: optimal parameters for alias-free, high sensitivity spectroscopic imaging. J Magn Reson Imaging. 2009;29(6):1375-1385.
7. Jackson JI, Meyer CH, Nishimura DG, Macovski A. Selection of a convolution function for Fourier inversion using gridding [computerised tomography application]. IEEE Trans Med Imaging. 1991;10(3):473-478.
8. Bucholz EK, Song J, Johnson GA, Hancu I. Multispectral imaging with three-dimensional rosette trajectories. Magn Reson Med. 2008;59(3):581-589.
9. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007;58(6):1182-1195.
10. Zhao W, Cohen-Adad J, Polimeni JR, Keil B, Guerin B, Setsompop K, Serano P, Mareyam A, Hoecht P, Wald LL. Nineteen-channel receive array and four-channel transmit array coil for cervical spinal cord imaging at 7T. Magn Reson Med. 2014;72(1):291-300.
Figures