1537
Pretherapeutic specific gray matter volume for predicting MRgFUS thalamotomy-mediated tremor response in Parkinson's disease1Department of Radiology, Chinese PLA General Hospital, Beijing, China
Synopsis
Keywords: Neurodegeneration, MR-Guided Interventions, MRgFUS
This is the first study to assess magnetic resonance-guided focused ultrasound (MRgFUS) VIM thalamotomy on gray matter (GM) volume in tremor-dominant Parkinson’s disease (PD). MRgFUS has good efficacy and safety in the treatment of PD. We used three methods to extract specific GM volumes of nine PD patients before and after treatment for calculating their difference and correlation analysis. We found that the specific GM regions may predict tremor responses in PD after thalamotomy, and the results help to better understand the distant effect of MRgFUS thalamotomy and the involvement of GM in tremor control in PD.Introduction
Magnetic resonance imaging-guided focused ultrasound (MRgFUS) is a novel and minimally invasive technology in the treatment of Parkinson’s disease (PD). However, the mechanism of MRgFUS-mediated tremor improvement is unclear. Recently, the regional differences in gray matter (GM) volume have been reported to be reliable for discriminating PD patients from healthy controls (HC) versus different subtypes [1-3]. Therefore, the aim of the present study was to investigate possible GM changes and their relationship with tremor symptoms from pre- and post-operation in PD. For this purpose, we performed a systematic whole-brain investigation of GM in cortical and subcortical structures and a correlation analysis between GM changes and tremor improvement in PD with MRgFUS thalamotomy.Method
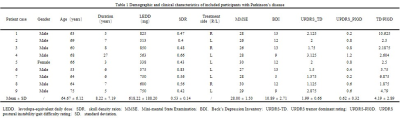
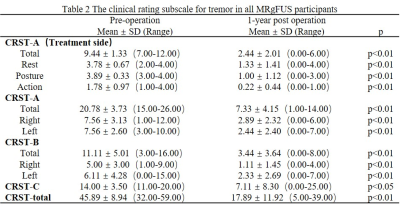
This study included 10 right-handed patients with tremor-dominant PD for MRgFUS thalamotomy targeting the VIM nucleus and nine age- and gender-matched right-handed HC, one of the patients was lost to follow-up after treatment. The study was conducted with the approval of the institutional ethics committee and subjects were recruited at the Chinese PLA General Hospital with written informed consent before the research (Clinical Trials.gov no. NCT04570046). The criteria for diagnosis of PD and surgery were consistent with previous reports [4]. Clinical evaluations were collected at baseline and 1-year follow-up. MRI data were collected at baseline, and 1 day, 7 days,1 month, 3 months, and 12 months after surgery. The demographic and clinical characteristics collected are summarized in Table 1,and Table 2. Patients were assessed for tremors with the Clinical Rating Scale for Tremor (CRST) in the off-medication state. All the participants underwent a standardized MRI protocol on a GE 3.0T suite. High-resolution three-dimensional T1-weighted (T1-3D) with fast spoiled gradient recalled was used for analysis, including the following parameters: repetition time [TR] = 6.656 msec, echo time [TE] = 2.928 msec, flip angle = 7°, field of view [FOV] = 256 mm, matrix =256 × 256, 192 contiguous sagittal 1-mm-thick slices. We used the Computational Anatomy Toolbox 12 software (CAT12) and SUIT toolbox (http://www.diedrichsenlab.org/imaging/suit_function.htm) running under SPM12 to obtain gray matter volumes and cerebellar volumes [1]. FIRST was used to estimate absolute volumes of subcortical structures [5]. For demographic and clinical information, the independent t-test and the χ2 test were used for the continuous variables and the dichotomous variables, respectively. Brain regions were reported at an initial threshold of voxel-wise p < 0.001 (uncorrected for multiple comparisons) with cluster size threshold of 20 contiguous voxels [6]. Importantly, we used the Anatomical Automatic Labeling (AAL) 90 atlas to extract the volume of the brain region of interest for correlation analysis with clinical scales. Besides, all figures were constructed based on SPMs at p < 0.001 (uncorrected for multiple comparisons) with a cluster forming threshold of approximately 100 voxels for visualization. Partial correlation analyses were used to assess the correlations between GM measures and clinical scores. The significance threshold was set at 0.05, two-tailed. Statistical analyses of all data were performed using SPSS 26.0 statistical software (http://www.spss.com/).Result
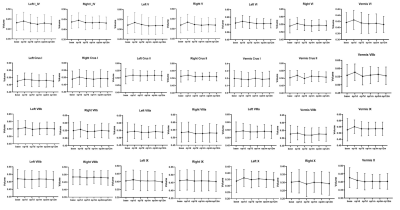
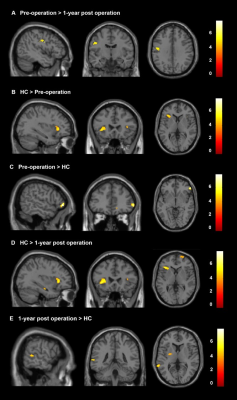
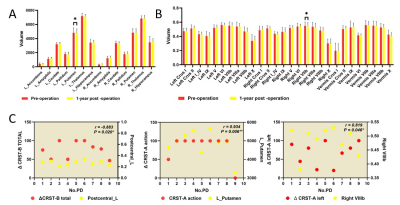
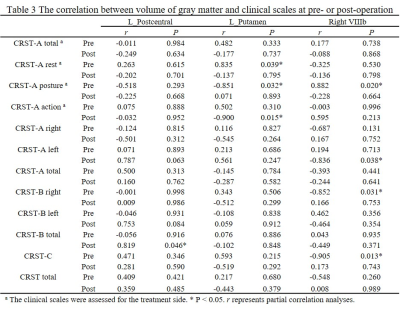
Age and gender did not differ significantly between HC and PD groups. Other demographic and clinical data are shown in Table 1 and Table 2. It can be seen from Figure 1 and Figure 2 that the GM subregions after MRgFUS treatment is in a dynamic change trend over time. Voxel-wise paired t test revealed a significant decrease of GM in the left left postcentral and left precentral when contrasting pre-operation versus 1-year post-operation in PD (Fig.3). After the 1-year follow-up, GM volume of left putamen (p = 0.041, Fig.4) and right cerebellar lobular VIIIb (p = 0.006, Fig.4) were significant reduction in PD, respectively. We did not find any significant differences in other GM subregions (Fig.4). The postcentral volume was negative correlation with tremor improvement (CRST-B total: r = -0.883, p = 0.020, Fig.4). Meantime, the volume of the left putamen and the right cerebellar lobule VIIIb were positive correlation with tremor improvement (action tremor: r = 0.934, p = 0.006; CRST-A: r = 0.819, p = 0.046, Fig.4).Discussion
This study revealed that MRgFUS had an effect on different GM volume showing a dynamic change process (Fig.1, Fig.2). PD patients at 1-year follow-up also exhibited widespread lower voxel in the left postcentral and the left precentral relative to pre-operation (Fig.3). Previous studies have also reported the role of the postcentral gyrus in PD [7, 8]. We also detect correlation between brain postcentral volume on the left and tremor improvement in PD patients (Table 3). Other findings that deserves a comment is that of GM abnormalities observed in the left putamen and specific cerebellum subregion in PD after MRgFUS thalamotomy which are correlation with tremor improvement (Fig.4), suggesting tremor improvement could modulate by MRgFUS thalamotomy acting on specific GM volume.Conclusion
GM volume showed dynamic change after thalamotomy. The specific GM region, for the first time, reported as relevant to tremor improvement in PD after MRgFUS thalamotomy, suggesting a distant effect of MRgFUS thalamotomy and the involvement of GM in tremor control in PD. Future studies with larger sample sizes are needed to investigate the potential role of these regions in predicting tremor improvement after thalamotomy.Acknowledgements
This work has been supported by the National Natural Science Foundation of China (Nos. 81825012, and 82151309). Xin Lou is the author who received the funding.References
[1] XU X, HAN Q, LIN J, et al. Grey matter abnormalities in Parkinson's disease: a voxel-wise meta-analysis [J]. European journal of neurology, 2020, 27(4): 653-9.
[2] SARASSO E, AGOSTA F, PIRAMIDE N, et al. Progression of grey and white matter brain damage in Parkinson's disease: a critical review of structural MRI literature [J]. Journal of neurology, 2021, 268(9): 3144-79.
[3] CHEN J, JIANG X, WU J, et al. Gray and white matter alterations in different predominant side and type of motor symptom in Parkinson's disease [J]. CNS neuroscience & therapeutics, 2022, 28(9): 1372-9.
[4] JANKOVIC J, MCDERMOTT M, CARTER J, et al. Variable expression of Parkinson's disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group [J]. Neurology, 1990, 40(10): 1529-34.
[5] PIETRACUPA S, BOLOGNA M, BHARTI K, et al. White matter rather than gray matter damage characterizes essential tremor [J]. European radiology, 2019, 29(12): 6634-42.
[6] PICCININ C C, CAMPOS L S, GUIMARãES R P, et al. Differential Pattern of Cerebellar Atrophy in Tremor-Predominant and Akinetic/Rigidity-Predominant Parkinson's Disease [J]. Cerebellum (London, England), 2017, 16(3): 623-8.
[7] SUO X, LEI D, LI N, et al. Disrupted morphological grey matter networks in early-stage Parkinson's disease [J]. Brain structure & function, 2021, 226(5): 1389-403.
[8] JI G J, HU P, LIU T T, et al. Functional Connectivity of the Corticobasal Ganglia-Thalamocortical Network in Parkinson Disease: A Systematic Review and Meta-Analysis with Cross-Validation [J]. Radiology, 2018, 287(3): 973-82.
Figures