1534
Brain alterations in ovariohysterectomized rats revealed by diffusion tensor imaging1Department of Biomedical Imaging and Radiological Science, China Medical University, Taichung, Taiwan, 2Institute of Biomedical Engineering and Nanomedicine, National Health Research Institutes, Miaoli, Taiwan
Synopsis
Keywords: Neurodegeneration, Diffusion Tensor Imaging
Women undergoing hysterectomy with oophorectomy have an increased risk of Alzheimer’s disease. However, postoperative neuroimaging data on pathogenic processes in the brain are limited. This study was aimed to investigate the potential effect of ovariohysterectomy on brain integrity in the rat model using diffusion tensor imaging technique. Compared to the control group, the ovariohysterectomy group showed significantly lower fractional anisotropy in the corpus callosum, bilateral striatum, and bilateral cortex, suggesting neuronal injury in ovariohysterectomized rats. Therefore, neuroimaging should be performed to monitor brain alterations in women after hysterectomy with bilateral oophorectomy in clinical settings.Introduction
Hysterectomy is a common gynecological surgery in women that is usually performed for benign conditions, such as irregular menstrual bleeding and uterine fibroids [1]. In addition, concomitant bilateral oophorectomy is performed in > 50% of these patients [1] to prevent progression to ovarian cancer. Despite the safety and benefits of hysterectomy with bilateral oophorectomy, attentions should be paid to the effect of surgery on long-term health in women. Recently, hysterectomy increases the risk for early-onset dementia, particularly when accompanied by oophorectomy preceding the onset of menopause [2]. Although brain function after hysterectomy with oophorectomy is aberrant, postoperative neuroimaging data on pathogenic processes in the brain are limited. Therefore, the aim of this study was to investigate the potential effect of hysterectomy with oophorectomy on brain integrity in a rat model using diffusion tensor imaging (DTI).Methods
Animal preparation: We enrolled 26 female Sprague-Dawley rats (7 weeks old), including 13 each in the control and ovariohysterectomy groups. A licensed veterinarian performed ovariohysterectomy via midline abdominal incision. MRI experiments: All rats underwent MRI experiments at 9 weeks of age using a 7T animal MRI scanner (Bruker ClinScan 70/30, Germany) with a gradient strength of 630 mT/m. DTI was performed using a single-shot echo planar imaging (EPI) sequence with the following parameters: repetition time/echo time = 6000 ms/32 ms, flip angle = 90°, field of view = 35 × 35 mm2, matrix size = 128 × 128, nine axial slices, thickness = 1.5 mm, 30 gradient directions, 4 b values of 0, 500, 1000, and 1500 s/mms, and three averages. Data analysis: The diffusion-weighted images were realigned to the non-diffusion-weighted b0 image. DSI Studio was used to estimate the DTI-derived parameters fractional anisotropy (FA) and mean diffusivity (MD). The regions-of-interest (ROIs) of the corpus callosum, bilateral striatum, and bilateral cortex were manually derived from EPI scans of each rat. The resulting ROI masks were then applied to the FA and MD maps to calculate regional values by averaging the values from all voxels in the ROI mask. Student’s t-test was applied to pairs of studied groups of regional FA and MD.Results
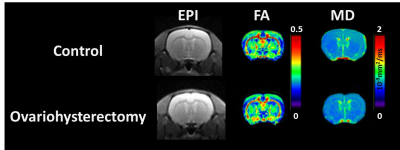
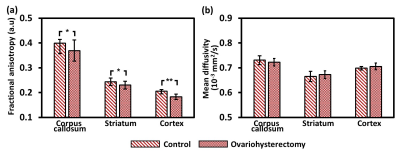
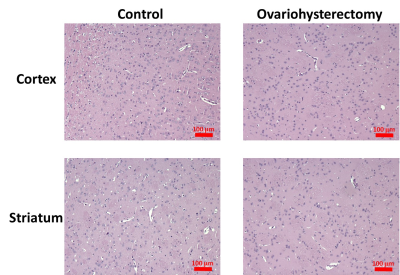
Figure 1 illustrates the typical EPI and FA and MD maps from representative rats in both groups. Visual inspection revealed lower FA in widespread brain regions of ovariohysterectomized rats than in those of control rats. Figure 2(a) and 2(b) show the results of the ROI analyses of FA and MD in the two groups. Two weeks after ovariohysterectomy, a two-sample t-test showed significant differences in FA between the two groups in all three brain regions (all P < 0.05), and ovariohysterectomized rats showed significantly lower FA values compared to control rats. MD did not differ between the two groups (all P > 0.05). Figure 3 shows light microscopic images of hematoxylin and eosin (H&E)–stained sections of the brain tissues from control and ovariohysterectomy groups. Compared to the control, most cells were irregular in shape with shrunken deeply stained nuclei in the ovariohysterectomy group. H&E staining demonstrated neuronal injury in the ovariohysterectomy group.Discussion and Conclusion
In the present study, brain alterations following ovariohysterectomy were assessed using the non-invasive DTI technique. The results demonstrated that rats have lower FA after ovariohysterectomy in widespread brain regions, suggesting neuronal injury and demyelination. When the myelin sheath is compromised or pyramidal neuron degenerated because of disease, FA decreases because of diffusion changes. Estrogen has neuroprotective effects, and estrogen deficiency after oophorectomy can reduce synapse formation in dendritic spines in the brain [3]. The decreased FA in several brain regions in rats after ovariohysterectomy in this study was consistent with previous studies, suggesting that reduced estrogen levels after surgery causes brain alterations. As pathological changes in several brain regions in rats after ovariohysterectomy (lower FA compared to control) resembled those reported for Alzheimer’s disease and Parkinson’s disease in clinical studies [4, 5], these findings highlight the requirement of long-term follow-up using DTI to monitor brain changes in women after hysterectomy or oophorectomy.Acknowledgements
No acknowledgement found.References
[1] L.S. Wilcox, L.M. Koonin, R. Pokras, L.T. Strauss, Z. Xia, H.B. Peterson, Hysterectomy in the United States, 1988-1990, Obstetrics and gynecology 83(4) (1994) 549-55.
[2] W.A. Rocca, J.H. Bower, D.M. Maraganore, J.E. Ahlskog, B.R. Grossardt, M. de Andrade, L.J. Melton, 3rd, Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause, Neurology 69(11) (2007) 1074-83.
[3] B.S. McEwen, S.E. Alves, Estrogen actions in the central nervous system, Endocrine reviews 20(3) (1999) 279-307.
[4] C.E. Sexton, U.G. Kalu, N. Filippini, C.E. Mackay, K.P. Ebmeier, A meta-analysis of diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease, Neurobiology of aging 32(12) (2011) 2322 e5-18.
[5] C.J. Cochrane, K.P. Ebmeier, Diffusion tensor imaging in parkinsonian syndromes: a systematic review and meta-analysis, Neurology 80(9) (2013) 857-64.
Figures