1532
Increase of Magnetic Transfer Contrast in Middle Cerebellum Peduncles in Patients with Multiple System Atrophy1Radiology, Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China, 2MR Collaborations, Siemens Healthineers Ltd., Shanghai, China, 3Ruijin Hospital / Luwan Branch Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
Synopsis
Keywords: Neurodegeneration, Magnetization transfer, Multiple system atrophy
The magnetic transfer (MT) MRI imaging has long been used for quantifying neuromelanin containing nucleus, but its ability of quantifying the magnetization exchange between free water and macromolecules has also allowed its potential usage in detecting white matter changes. Patients with multiple system atrophy has unique pathological changes in pontocerebellar regions. In this study, we have not only found significant increase of MT contrast in MCP regions of MSA, but also proved significant correlation between this alteration and brainstem or cerebellar atrophy, which might offer new insights for future studies.Introduction
MRI technique with magnetic transfer (MT) contrast has been widely used to investigate neuromelanin contents in nucleus such as substantia nigral and locus coeruleus in many neurogenerative disorders [1-3]. Pathologically, in addition to the neuronal loss and gliosis in pontine nucleus, the atrophy in olivopontocerebellar regions and significant myelin pallor and demyelination in the cerebellar white matter are also found in patients with multiple system atrophy (MSA) [4]. Magnetization transfer imaging has the ability to characterizes the amount and degree of magnetization exchange between free water and macromolecules like proteins in the myelin bilayers [5, 6]. However, the MTC signal changes due to aberrances of the pontocerebellar regions such as the middle cerebellum peduncles have remained unclear.Purpose
This study aimed to assess if there were significant differences of MCP MT contrast between MSA and healthy controls (HCs) and the underlying mechanism of it.Methods
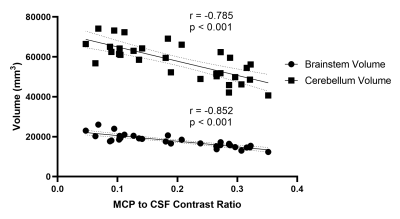
19 patients who were diagnosed as MSA according to international diagnostic criteria [7] during hospital stay were consecutively recruited to this study. MSA patients were also classified into two clinical phenotypes: MSA with predominant parkinsonism (MSA-P) and MSA with predominant cerebellar ataxia (MSA-C) according to their predominant motor symptom [7] (12 MSA-C, and 7 MSA-P patients in our study). 12 age- and gender- matched HCs were also recruited during the study. Before MRI acquisition, the Unified MSA Rating Scale (UMSARS) part IV representing clinical disability was assessed by experienced researchers. MRI scans were performed on a 3T MRI scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany), including a T1-weighted 3-dimensional magnetization-prepared rapid gradient echo (T1 3D MPRAGE) sequence for brain parcellation and a 2D GRE sequence with magnetization transfer contrast preparation pulse imaging for quantifying MCP contrast. Freesurfer version 7.2.0 was used to segment and calculate the volumes (mm3) of the brainstem and cerebellum for each subject. Signal intensity (SI) of bilateral MCPs and reference region of cerebrospinal fluid (CSF) on the same layer near the MCPs were measured by manually drawn ROIs. MCP to CSF contrast ratio were then calculated as MCPcontrast ratio = [(SIMCP – SICSF)/SICSF]. Comparisons of MCP contrast and brainstem and cerebellum volume between MSA and HC groups were made by using two-sample T test or Mann-Whitney U test based on distribution of the statistics, and the differences among HC, MSA-P, MSA-C groups were analyzed using one-way ANOVA and post-hoc analyses were further performed using least-significant difference (LSD) method. Spearman’s correlations between MCP contrast and UMSARS-IV rating scale were also performed.Results
In MSA patients, MCP contrast ratio was significantly increased (P<0.001) and the brainstem volume was significantly reduced (P < 0.001) compared with HC. And compared with MSA-P, significant increase of MCP contrast was observed in patients with MSA-C (P = 0.001), as well as significant reduction of brainstem volume in MSA-C (P = 0.03). And we also found significant negative correlations between MCP contrast ration and brainstem volume (R = -0.852, P <0.001), and between MCP contrast ratio and cerebellum volume (R = -0.785, p<0.001). Moreover, the MCP MT contrast has demonstrated significant positive correlation with UMSARS-IV rating scales (Rho = 0.749, P = 0.008) in MSA-C subgroup. However, no significant correlation between MCP contrast ration and UMSARS-IV was found in MSA-P subgroupDiscussion
By adapting Magnetization transfer imaging, we have discovered the increase of MCP MT contrast in patients with MSA and this was in significant correlation with brainstem and cerebellar volume reduction. This discovery offered new insights for white matter changes in olivopontocerebellar regions of MSA from the perspective of MT imaging, and it might help diagnosis and differential diagnosis of MSA. We further analyzed correlations between MCP contrast and rating scales of clinical disability in MSA-C and MSA-P. We reported that MCP contrast tended to be higher as the overall disability getting more severe (representing a higher UMSARS-IV scale) in MSA-C patients.Conlusion
MSA patients have significantly increased MCP MT contrast compared to HC. As pontocerebellar region atrophy getting more severe, the contrast ratio of MCP on MT imaging increases accordingly. In MSA-C patients, the MCP contrast tended to be higher as the overall disability getting more severe.Acknowledgements
The authors thank the patients and their families for the time and effort they dedicated to the research. This study has received funding from National Natural Science Foundation of China (82171891, 81901694), and Scientific Research Program of Shanghai Science and Technology Commission of China (21ZR1439800).References
[1] Priovoulos N, van Boxel SCJ, Jacobs HIL, et al. Unraveling the contributions to the neuromelanin-MRI contrast. Brain Struct Funct. 2020;225(9):2757-2774. doi:10.1007/s00429-020-02153-z
[2] He N, Chen Y, LeWitt PA, Yan F, Haacke EM. Application of Neuromelanin MR Imaging in Parkinson Disease [published online ahead of print, 2022 Aug 26]. J Magn Reson Imaging. 2022;10.1002/jmri.28414. doi:10.1002/jmri.28414
[3] Seiler S, Ropele S, Schmidt R. Magnetization transfer imaging for in vivo detection of microstructural tissue changes in aging and dementia: a short literature review. J Alzheimers Dis. 2014;42 Suppl 3:S229-S237. doi:10.3233/JAD-132750
[4] Koga S, Dickson DW. Recent advances in neuropathology, biomarkers and therapeutic approach of multiple system atrophy. J Neurol Neurosurg Psychiatry. 2018;89(2):175-184. doi:10.1136/jnnp-2017-315813
[5] Sled JG. Modelling and interpretation of magnetization transfer imaging in the brain. Neuroimage. 2018;182:128-135. doi:10.1016/j.neuroimage.2017.11.065
[6] Alexander AL, Hurley SA, Samsonov AA, et al. Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connect. 2011;1(6):423-446. doi:10.1089/brain.2011.0071
[7] Wenning GK, Stankovic I, Vignatelli L, et al. The Movement Disorder Society Criteria for the Diagnosis of Multiple System Atrophy. Mov Disord. 2022;37(6):1131-1148. doi:10.1002/mds.29005