1531
Depression symptoms in Parkinson's disease correlate with amygdala subregions atrophy1The First Affiliated Hospital of Dalian Medical University, Dalian, China, 2GE Healthcare, Shanghai, China
Synopsis
Keywords: Neurodegeneration, Psychiatric Disorders
Depression is the most frequent psychiatric disorder in Parkinson's disease (PD). Amygdala pathology has been suggested to contribute to some clinical features of PD, including deficits of olfaction and mood disorders. We aimed to more accurately measure alterations in the volume of each amygdala nucleus in Parkinson's disease with depression (DPD) patients. Then the volume of each specific amygdala nucleus would be associated with the severity of depressive symptoms. This study showed that DPD patients had multiple amygdala subregions atrophy. The bilateral lateral amygdala and left accessory basal nucleus were negatively correlated with the severity of depression in DPD patients.Summary of Main Findings
This study explored the subregional atrophy pattern of the amygdala in Parkinson's disease with depression (DPD) patients and its correlation with the severity of depressive symptoms. The results showed that DPD patients had multiple amygdala subregions atrophy, especially in the bilateral lateral nucleus, left accessory basal nucleus, right cortical nucleus, right central nucleus, and right medial nucleus. Partial correlation analysis showed that the bilateral lateral amygdala and left accessory basal nucleus were negatively correlated with the severity of depression in DPD patients.Introduction
Depression is the most common psychiatric disease in Parkinson's disease (PD), occurring in approximately half of the patients[1]. Lewy body accumulation in specific brain areas of PD patients may damage emotion-related functions, leading to depression. Among these areas, the amygdala may present with the earliest to be damaged in PD[2]. To be more specific about the amygdala structure, it is composed of interconnected substructures with distinct histological features. Total amygdala volumetric measurements do not reflect subtle volumetric changes in each subnucleus of the amygdala. However, there are almost no studies on changes in amygdala subregion volumes in DPD patients. By looking into amygdala subregion alterations in DPD patients and their relationship to depression, we hoped to find a possible pathological link between the two diseases.Materials and Methods
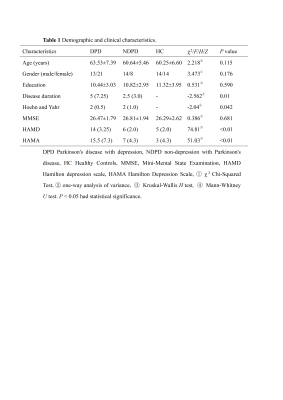
This study included 56 patients with PD (34 in the DPD group, 22 in the NDPD group) and 28 healthy controls (HC) matched by sex, age and education. We used the Hamilton Depression Scale (HAMD), with a HAMD score of >7 in the DPD group and a HAMD score of ≤7 in the non-depression with Parkinson's disease (NDPD) group. All Participants were scanned using a 3.0 T GE Signa HDXT scanner from America equipped with an 8-channel head coil. A 3D magnetization-prepared rapid-acquisition gradient-echo T1-weighted sequence with the following parameters was performed: repetition time = 10.2 ms, echo time (shortest) = 4.2 ms, flip angle = 13°, FOV = 24 × 24cm2, matrix size = 256 × 256, voxel size = 1.0 × 1.0 × 1.0 mm, slice thickness 1.0 mm. Segmentation of amygdala subregions was performed using FreeSurfer 6.0 software. The amygdala was divided into 9 subregions: lateral nucleus, basal nucleus, accessory basal nucleus, anterior amygdaloid area, central nucleus, medial nucleus, cortical nucleus, cortico-amygdaloid transition area, and paralaminar nucleus. The estimated total intracranial volume (eTIV) volumes were also extracted. Statistical analyses were performed using SPSS 26.0 software. Covariance analysis (ANOVA) was applied to compare the amygdala subregion volume differences among the three groups. Partial correlation analysis was performed to evaluate the correlation between significantly reduced amygdala subregion volumes and the severity of depressive symptoms. Age, gender, education, and eTIV were included as covariates. The FDR was used for correction.Result
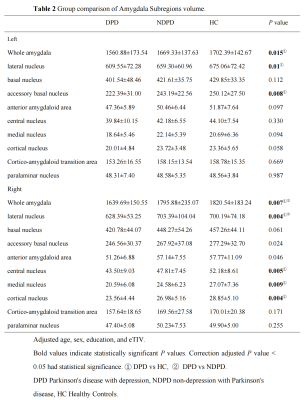
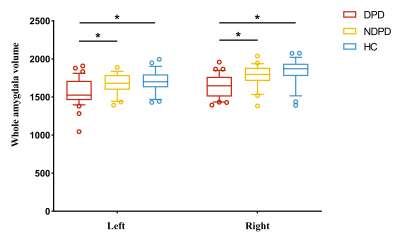
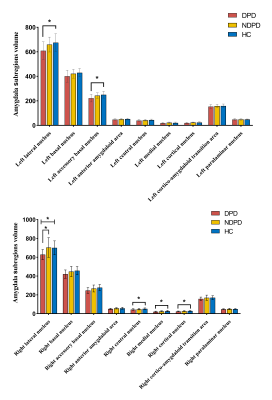
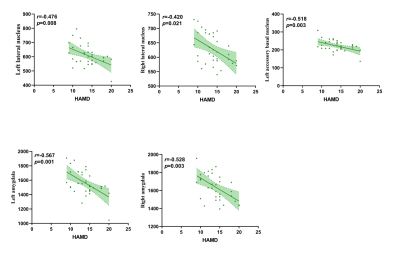
The demographic information and clinical data are summarized in Table 1. As shown in Fig. 1. We found that the bilateral total amygdala volumes were significantly lower in the DPD group than in the NDPD group and HC group (left P= 0.015 and right P= 0.007). Further comparing the volumes of bilateral amygdala subregions, we found that the volume of the DPD group was significantly lower than that of the HC group, which were in the bilateral lateral nuclei (left P= 0.016, right P= 0.019), left accessory basal nucleus (P= 0.029), right cortical nucleus (P= 0.004), right central nucleus (P= 0.004), and right medial nucleus (P= 0.007). However, Compared with the NDPD group, the DPD group showed a significantly lower volume in the right lateral nucleus (P= 0.009) (Table 2, Fig. 2). In the DPD group, partial correlation analysis showed that the bilateral lateral nuclei (left r= -0.476, P= 0.008; right r= -0.420, P= 0.021) and left accessory basal nucleus (r= -0.518, P= 0.003) were negatively correlated with HAMD scores (Fig. 3).Discussion
Different subregions of amygdala have different degrees of morphometric vulnerability to DPD. We observed the reduced volumes of the bilateral lateral nuclei, left accessory basal nucleus, right cortical nucleus, right central nucleus, and right medial nucleus in patients with DPD. Atrophy of the lateral nucleus, the largest subregion of the amygdala, may reduce the emotional memory-enhancing effects associated with sensory stimuli[3] and impair emotion regulation. The central and medial nuclei are the main output nuclei of the hypothalamus, and both are particularly sensitive to negative emotional stimuli[4]. The cortical nucleus has a major connection with olfactory-associated brain regions, receiving significant input from the olfactory bulb[5]. Its degeneration may contribute to the common early dysosmia in PD.Conclusion
This study suggests that morphological changes in the amygdala subregion may underlie the occurrence of DPD. DPD patients have multiple amygdala subregional atrophy. Moreover, the aggravation of depressive symptoms was closely correlated with the bilateral lateral nuclei and left accessory basal nucleus.Acknowledgements
No acknowledgement found.References
[1] Dooneef G, Mirabello E, Bell K, Marder K, Stern Y, Mayeux R. An estimate of the incidence of depression in idiopathic Parkinson’s disease. Arch Neurol 1992;49:305–307.
[2] Braak H, Braak E, Yilmazer D, de Vos RA, Jansen EN, Bohl J, Jellinger K. Amygdala pathology in Parkinson's disease. Acta Neuropathol. 1994;88(6):493-500.
[3] Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learn Mem. 2001 Sep-Oct;8(5):229-42.
[4] Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001 Jan;6(1):13-34.
[5] Swanson LW, Petrovich GD. What is the amygdala? [Review]. Trends Neurosci 1998; 21: 323–331.
Figures