1529
Mapping neuromelanin loss in clinically uncertain parkinsonism differentiates neurodegenerative from non-neurodegenerative1Mental Health and Clinical Neuroscience Unit, University of Nottingham, Nottingham, United Kingdom, 2Sir Peter Mansfield Imaging Centre, University of Nottingham, Nottingham, United Kingdom, 3NIHR Nottingham Biomedical Research Centre, University of Nottingham, Nottingham, United Kingdom, 4Nottingham University Hospitals, Nottingham, United Kingdom, 5Department of Radiology, Cardiff and Vale University, Cardiff, United Kingdom
Synopsis
Keywords: Neurodegeneration, Neurodegeneration, clinical uncertain Parkinsonism, NM-MRI
Brain dopamine transporter SPECT imaging is routinely used to assess striatal dopaminergic deficit in the differentiation of essential tremor from degenerative parkinsonism in clinically uncertain parkinsonism (CUP). Neuromelanin (NM)-MRI detects nigral depigmentation with good diagnostic accuracy in confirmed Parkinson’s Disease; but its diagnostic value in CUP is unclear. Using voxel-based analysis following optimised automatic registration, we investigated the topography of NM loss in CUP, and show that voxels that best discriminate between non-neurodegenerative and neurodegenerative CUP are co-located in the dorsolateral substantia nigra.Introduction:
Parkinson’s disease (PD) is the second most common neurodegenerative disease. Its diagnosis in patients with some motor symptoms of PD lookalikes can be difficult, especially at early stage (clinically uncertain parkinsonism, CUP). The dopamine transporter (DAT)-specific PET/SPECT scanning (DATscan) of the striatum has been used to assistant the diagnosis, but it has several shortcomings1. Contrary to DATscan, MRI is not radioactive and more widely available. In addition, increasing evidence has shown that imaging of the substantia nigra pars compacta (SNpc) with neuromelanin-sensitive MR (NM-MRI) offers high diagnostic accuracy to distinguish patients with established PD and healthy controls2-3. However, it is still unknown whether NM-MRI can prospectively provide adjunct to the clinical differential diagnosis of CUP. Methodologically, most previous studies calculated region of interest-based contrast or thresholding dependent volumetry metrics4-5. As an alternative to these measures, voxelwise based analysis was validated to provide a more precise substructural-specific topography of the SNpc6. Therefore, in this study, we applied voxelwise approach to a cohort of patients with CUP, aiming to investigate whether and how NM-MRI enables discrimination of PD from other non-dopamine deficiency aetiologies of Parkinsonism.Methods:
Subjects:A total of 111 CUP participants as a multi-centre longitudinal imaging study (N3iPD – https://clinicaltrials.gov/ct2/show/NCT03022357). Of these, 119 participants had complete set of UPDRS-III scores, with 74 classed as CUP-PD (44 males; age mean±std 67±11 years) and 45 as CUP-non-NP (24 males; age: 69±10 years) at the clinical follow-up visit.Neuromelanin MRI protocols:
-Nottingham: 3T GE and 32-channel head coil was used for the MRI acquisition. NM-MRI was acquired with 3D‐dimensional spoiled gradient recalled T1‐weighted sequence.
-London: 3T Siemens and 8-channel head coil was used. T1-weighted turbo spin echo sequence was applied to acquire NM-MRI.
NM-MRI processing and analysis:
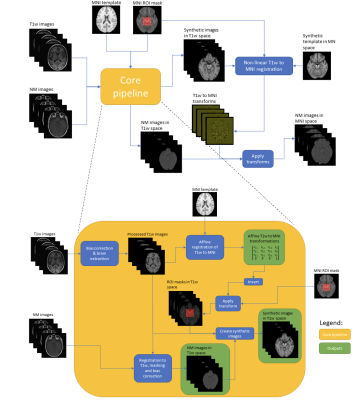
All the NM images passed quality control. MR images were processed following a synthetic normative template-based in-house automated pipeline (Figure 1, method details in7). As a result, all the NM images were co-registered to the 1mm isotropic MNI space. Intensity normalisations and the voxelwise analysis were performed using in-house Matlab (Mathworks) scripts. A substantia nigra mask and a brainstem mask manually drawn in the synthetic template, were used to derive the background (BS) region (brainstem area-SN regions)3. The intensity of each voxel in the brainstem was normalised by dividing the median of BS. To identify voxels that are associated with between-group discrimination (CUP-PD vs. CUP-nonPD), a binary logistic regression analysis was conducted as follows: G=b0+b1´NormI+b2´sex+b3´age+b4´site+e, where G is the grouping, Norml is the normalised voxel intensity, and sex, age and scanning site were nuisance variables. T values of the voxels, which reflect the size of influence of grouping on normalised voxel intensity of the BS were illustrated as a topography. To validate the t-value map, we randomised the group labelling, and then repeated the same regression analysis. The resulting trandom map was plotted. Finally, to locate the voxels correlated with motor impairment severity, we also performed partial correlation between normalised voxel intensity and MDS-UPDRS-III scores by regressing out sex, age and scanning site. [Figure 1]
Results:
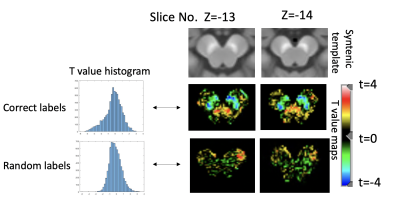
Our regression analysis showed that the lower normalised voxels intensity in SN regions were significantly associated with CUP-PD, indexed by negative t values, in contrast to the voxels in the remaining BS regions (illustrated as blue areas in Figure 2 middle row on the right). In addition, our finding revealed that voxels with higher influence (darker blue voxels representing lower t values) on the discrimination of CUP-nonPD vs PD was in the dorsolateral part of both SNs. This t-value map is markedly different from the map using the randomisation approach (Figure 2 bottom row), which were also presented as histograms (Figure 2 left panel). Yet, the present study did not find any voxels significantly correlated with motor impairment severity. [Figure 2]Discussion:
With our automatic co-registration method and the voxelwise approach, we investigated whether and how NM enables the identification of voxels that are associated with CUP discrimination. In this study, we for the first time, revealed the brainstem topography that contribute to the group difference of CUP-PD and CUP-nonPD. Specifically, we prospectively identified the distribution of depigmented voxels associated with CUP-PD vs CUP-nonPD using NM-MRI. The clinical diagnosis of CUP-nonPD at the follow-up in our study included be essential tremor, drug-induced, and vascular parkinsonism. This is in line with the findings from previous retrospective studies, which separately showed NM-MRI could be used as an imaging biomarker to distinguish cases with these non-neurodegenerative parkinsonism disorders from patients with idiopathic PD, using regional-based NM metrics8-9 (also see review 10). This study also showed that the voxels concentrated in the ventral SN-nigrosomal region could best discriminate between CUP-PD and CUP-nonPD (as darker blue color shown). This concords with the expected PD pathology (nigral depigmentation) and spatio-temporal progression of depigmentation along the ventral->dorsal SNpc axis, similar to previous evidence3,6. However, no association between voxel-wise NM-contrast and motor severity was found, which may be linked to the high thresholding phenomenon of clinical parkinsonian motor symptoms (see review11).Acknowledgements
The Michael J. Fox Foundation for funding this project. NIHR Nottingham Biomedical Research Centre grant for funding Stefan Pszczolkowski. Weston Brain Institute for funding Yue Xing and the development of the automatic registration pipeline.References
1. Schwarz, S. T., Xing, Y., Naidu, S., et al. Protocol of a single group prospective observational study on the diagnostic value of 3T susceptibility weighted MRI of nigrosome-1 in patients with parkinsonian symptoms: the N3iPD study (nigrosomal iron imaging in Parkinson's disease). BMJ open. 2017; 7(12), e016904.
2. Prasad, S., Stezin, A., Lenka, A., et al. Three-dimensional neuromelanin-sensitive magnetic resonance imaging of the substantia nigra in Parkinson's disease. European journal of neurology. 2018; 25(4), 680–686.
3. Xing, Y., Sapuan, A. H., Martín-Bastida, A., et al. Neuromelanin-MRI to Quantify and Track Nigral Depigmentation in Parkinson's Disease: A Multicenter Longitudinal Study Using Template-Based Standardized Analysis. Movement disorders : official journal of the Movement Disorder Society. 2022; 37(5), 1028–1039.
4. Schwarz, S. T., Xing, Y., Tomar, P., et al. In Vivo Assessment of Brainstem Depigmentation in Parkinson Disease: Potential as a Severity Marker for Multicenter Studies. Radiology. 2017; 283(3), 789–798.
5. Wang, J. , Li, Y. , Huang, Z. , Wan, W., et al. Neuromelanin‐sensitive magnetic resonance imaging features of the substantia nigra and locus coeruleus in de novo Parkinson's disease and its phenotypes. European Journal of Neurology. 2018; 25(7), 949–e973.
6. Cassidy CM, Zucca FA, Girgis RR, et al. Neuromelanin-sensitive MRI as a noninvasive proxy measure of dopamine function in the human brain. Proc. Natl. Acad. Sci 2019; 116, 5108–5117.
7. Stefan Pszczolkowski, Tayyib T.A. Hayat, Yue Xing, et al. A synthetic normative template for Parkinson’s disease studies using structural and neuromelanin-sensitive magnetic resonance imaging, 18th International symposium on medical information processing and analysis. Valparaiso, 9-11 of November 2022
8. Reimão S, Pita Lobo P, Neutel D, et al. Substantia nigra neuromelanin-MR imaging differentiates essential tremor from Parkinson's disease. Mov Disord. 2015;30(7):953-959.
9. Matsuura K, Ii Y, Maeda M, et al. Neuromelanin-sensitive magnetic resonance imaging in disease differentiation for parkinsonism or neurodegenerative disease affecting the basal ganglia. Parkinsonism Relat Disord. 2021;87:75-81.
10. Pavese N, Tai YF. Nigrosome Imaging and Neuromelanin Sensitive MRI in Diagnostic Evaluation of Parkinsonism. Mov Disord Clin Pract. 2018;5(2):131-140.
11. Vermeiren Y, Hirschberg Y, Mertens I, De Deyn PP. Biofluid Markers for Prodromal Parkinson's Disease: Evidence From a Catecholaminergic Perspective. Front Neurol. 2020;11:595.
Figures