1527
Characterization of white matter microstructural abnormalities in cerebral small vessel disease with cerebral microbleeds1Department of Radiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China, 2Key Laboratory of Cognition and Personality (Ministry of Education); School of Psychology, Southwest University, Chongqing, China, 3Research Center for Brain-inspired Intelligence Institute of Automation, Chinese Academy of Sciences, Beijing, China, 4Department of Radiology, Shandong Provincial Hospital, Shandong University, Jinan, China, 5Department of Radiology, Beijing Friendship Hospital, Capital Medical University, Bijing, China
Synopsis
Keywords: Neurodegeneration, White Matter
To characterize white matter (WM) microstructural abnormalities in patients with cerebral small vessel disease (CSVD) coexisting with cerebral microbleeds (CMBs) and to further investigate the exact mechanism by which CMBs influence cognitive decline in patients with CSVD at the group and individual levels. Fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD) images from 49 CSVD patients with CMBs (CSVD-c), 114 CSVD patients without CMBs (CSVD-n), and 83 controls were analyzed using DTI-derived tract-based spatial statistics to detect WM diffusion changes among groups.Abstract
IntroductionCerebral small vessel disease (CSVD) is a disease of the small vessels in the brain 1. Cerebral microbleeds (CMBs) have been recognized to play a synergistic role in both cerebrovascular and neurodegenerative pathology occurring in the aging brain2. However, the exact mechanism by which CMBs influence cognitive decline severity in CSVD remains unclear, and further attention should be given to this topic. DTI can detect subtle microstructural damage to white matter (WM)3. However, until now, few studies have been carried out to quantify DTI-derived WM damage caused by CMBs in CSVD patients. Therefore, in this study, we used the DTI technique to explore the relationship between WM microstructural abnormalities and cognitive dysfunction in CSVD-c patients. The goal of this study was to characterize WM microstructural abnormalities using multiple diffusion indexes from DTI, investigate the correlations between diffusion changes and cognitive dysfunctions in CSVD patients with CMBs, and conduct individual prediction and identify discriminative features using multivariate pattern analysis.
Methods
This was a cross-sectional study approved by the institutional review board of Shandong Provincial Hospital Affiliated to Shandong First Medical University. 49 CSVD patients with CMBs, 114 CSVD patients without CMBs and 83 age- and sex-matched healthy subjects were recruited. All participants were evaluated by the neuropsychological scale and were imaged on a MAGNETOM Skyra 3.0 T MR scanner. Diffusion weighted images (DWIs) were acquired using a simultaneous multislice (SMS) accelerated single-shot echo planar imaging (EPI) sequence. T2-weighted (T2W) turbo spin echo, T2W fluid attenuated inversion recovery (FLAIR), T1-weighted (T1W) magnetization prepared rapid gradient echo (MPRAGE) and SWI scans were acquired to detect brain abnormalities. Fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD) images were analyzed from all participants using DTI-derived tract-based spatial statistics to detect WM diffusion changes among groups. Pearson’s correlations between regional diffusion changes and cognitive performance were investigated for all groups. Machine learning and multivariate pattern analysis (MVPA) were applied for group classification and identifying the discriminative WM diffusion features for predicting CSVD with CMBs.
Results
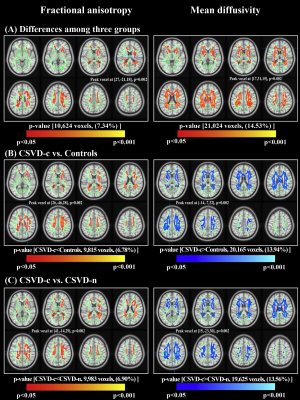
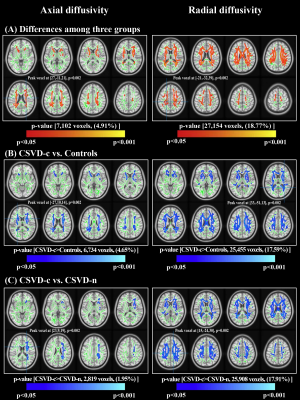
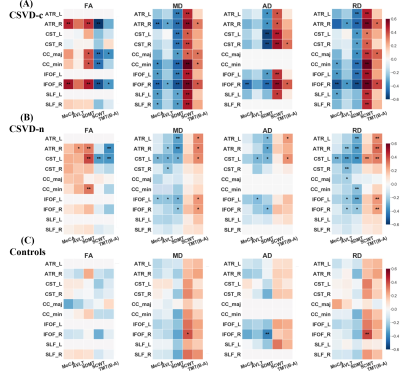
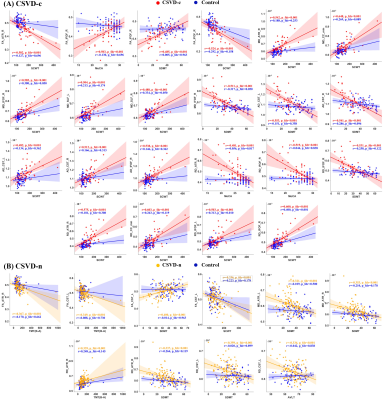
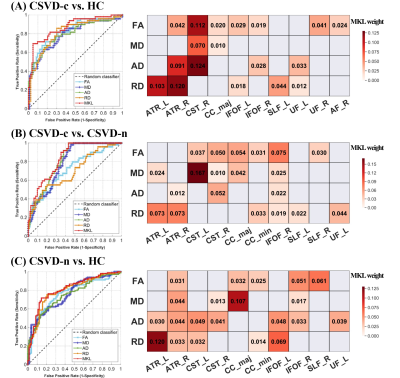
Compared with the CSVD-n and control groups, the CSVD-c group showed a significant FA decrease and AD, RD and MD increases mainly in the cognitive and sensorimotor-related WM tracts (Figure 1 and 2). Furthermore, the widespread regional diffusion alterations among groups were significantly correlated with cognitive parameters in both the CSVD-c and CSVD-n groups (Figure 3 and 4). Notably, we applied the multiple kernel learning technique in multivariate pattern analysis to combine multiregion and multiparameter diffusion features, yielding an average accuracy >77% for three binary classifications, which showed a considerable improvement over the single modality approach (Figure 5).
Discussion
The current study combined TBSS and MVPA methods to explore the WM microstructural damage caused by CMBs in CSVD patients and explored the correlation between WM microstructural damage and cognitive dysfunction in CSVD patients with or without CMBs. The main findings were as follows: (a) Changes in diffusion parameters were detected in many WM regions of the CSVD-c group, especially the cognitive and sensorimotor-related WM tracts. (b) The average FA, AD, RD and MD values of disrupted WM regions in the CSVD-c group were significantly correlated with cognitive parameters that were related to auditory verbal, symbol digit and executive control, and the significant correlations were stronger than those in the CSVD-n group. (c) CSVD-c patients could be differentiated from controls with high accuracy (85.61%, p<0.01) using the MVPA and MKL model combining multiregion and multiparameter diffusion features. These findings might help improve the present understanding of the neural mechanism of cognitive dysfunction in CSVD-c patients. Compared with the CSVD-n and control groups, the CSVD-c group showed widespread WM microstructural alterations characterized by decreased FA and increased AD, RD, and MD, mainly involving the cognitive and sensorimotor-related WM tracts. In addition, there was no significant difference in any diffusion metric between the CSVD-n and control groups, which suggested that the presence or absence of CMBs in CSVD patients has a great influence on the changes in WM. The existence of CMBs may aggravate the damage to WM tracts, resulting in more serious cognitive dysfunction in CSVD patients; this was an important finding of our study. Considering that previous studies have shown that the loss of WM microstructural integrity in specific regions was related to specific cognitive dysfunction in CSVD4, we further analyzed the correlations between diffusion metrics in disrupted WM regions and cognitive function in all groups. The MoCA, AVLT and SDMT scores in both CSVD groups were lower than those in the control group, while the SCWT and TMT (B-A) scores were higher than those in the control group, suggesting that these related cognitive functions in CSVD patients were significantly impaired. In addition, we combined MVPA with the MKL framework to effectively improve the classification accuracy, and more regions with WM damage were identified in our study than in the univariate analyses.
Conclusion
In conclusion, our study showed widespread WM microstructural damage in CSVD patients with CMBs and found that CSVD-c patients who had more severe white matter damage had lower cognitive function, which helped us to understand the mechanism of cognitive impairment in CSVD-c patients.
Acknowledgements
We thank all of the volunteers and patients for their participation in our study.References
1 Taylor-Bateman, V. et al. Cardiovascular Risk Factors and MRI Markers of Cerebral Small Vessel Disease: A Mendelian Randomization Study. Neurology, doi:10.1212/wnl.0000000000013120 (2021).
2 Li, J., Nguyen, T. D., Zhang, Q., Guo, L. & Wang, Y. Cerebral Microbleeds Are Associated With Increased Brain Iron and Cognitive Impairment in Patients With Cerebral Small Vessel Disease: A Quantitative Susceptibility Mapping Study. J Magn Reson Imaging 56, 904-914, doi:10.1002/jmri.28092 (2022).
3 Xu, Q. et al. Diffusion tensor imaging changes correlate with cognition better than conventional MRI findings in patients with subcortical ischemic vascular disease. Dement Geriatr Cogn Disord 30, 317-326, doi:10.1159/000320491 (2010).
4 Papma, J. M. et al. Cerebral small vessel disease affects white matter microstructure in mild cognitive impairment. Hum Brain Mapp 35, 2836-2851, doi:10.1002/hbm.22370 (2014).
Figures