1523
Pathological changes of the coronary microvascular dysfunction pig models based on diffuse tensor imaging (DTI)1Department of Radiology, Xijing Hospital, Fourth Military Medical University, Xi'an, China, 2Department of Radiology, Xijing Hospital, Forth Military Medical University, Xi'an, China, 3Philips Healthcare China, Xi'an, China, 4Xijing Hospital, Fourth Military Medical University, Xi'an, China
Synopsis
Keywords: Heart, Animals
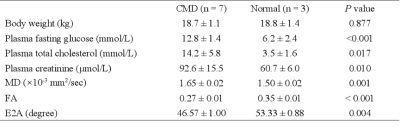
To investigate whether diffuse tensor imaging (DTI) can be used as a predictor for coronary microvascular dysfunction (CMD). Pig models with CMD had higher mean diffusivity (MD), lower FA, and lower E2A compared with normal pigs (mean MDnormal = 1.50 ± 0.02, mean MDCMD = 1.65 ± 0.02, P = 0.001; mean FAnormal = 0.35 ± 0.01, mean FACMD = 0.27 ± 0.01, P < 0.001; mean E2Anormal = 53.33 ± 0.88, mean E2ACMD = 46.57 ± 1, P = 0.004). Cardiac DTI technology could add benefits of offering microstructure change and cardiac remodeling parameters for CMD pig models.Introduction
In healthy myocardium, cardiomyocytes aggregate together to form laminar secondary structures known as sheetlets. Reorientation of sheetlets throughout the cardiac cycle is thought to be the principal mechanism driving LV wall thickening during systole (1). However, the impact of ischemic injury on sheetlets has not been explored in detail, especially for coronary microvascular dysfunction (CMD). MRI diffusion-weighted imaging (DWI) could identify the organization and integrity of micro-structural components, but the application in myocardium is limited due to artifact. Moreover, cardiac diffusion tensor imaging (cDTI) combined with second-order motion-compensated spin echo (M2SE) could acquire stable image quality(2). Based on the principle that water diffusion occurs preferentially along the long axis of cardiomyocytes, cDTI can provide information about the predominant orientations of cardiomyocytes and sheetlets within the myocardium (3). Previous cDTI studies on human (4-6) hearts have shown an increase in mean diffusivity (MD) and a decrease in the anisotropy of diffusion of water molecules defined by fractional anisotropy (FA) immediately after myocardial infarction. In cDTI, the secondary eigenvector angle (E2A) is a proposed measurement of the orientation of laminar sheetlets. The dynamic rearrangement of E2A between diastole and systole has been demonstrated in healthy participants, whereas low E2A can be used to explain the mechanistic deficiencies in wall strain and to predict LV remodeling in participants with dilated cardiomyopathy (1). Studies exploring the regional impact of myocardial infarction on E2A and its relationship with long-term remodeling are lacking. Therefore, cDTI sequence was performed in pig models to assess changes of myocardial microstructure.Method
Pig models were performed on a 3.0T MR platform (Ingenia CX, Philips Healthcare, the Netherlands). cDTI data were acquired using an electrocardiography-gated second-order motion-compensated (M2SE) single-shot spin-echo echo planar imaging sequence with asymmetric bipolar diffusion waveforms (7) and respiratory navigator tracking with the following parameters: repetition time of three R-R intervals, echo time of 89 msec; flip angle, 90°; field of view, 238 × 238 mm; matrix, 108 × 105; acquired in-plane resolution, 2.20 × 2.27; section gap, 8 mm; reconstructed voxel size, 1.7 × 1.7 × 8 mm; sensitivity encoding acceleration, 1.8; with a mean acquisition time of 13 minutes. Each cDTI data set constituted 18 noncollinear diffusion-weighted acquisitions with b values of 100 sec/mm2 (three acquisitions), 200 sec/mm2 (three acquisitions), and 500 sec/mm2 (twelve acquisitions). Based on cine data, trigger delay was set individually for each participant to coincide with 60% peak systole, and the center of k-space was approximately at 85% of peak systole. cDTI data post-processing was performed with in-house–developed Matlab software (Mathworks, Mathworks, USA ). Tensor eigenvalues, MD, FA, and E2A maps were calculated according to the tensors derived from diffusion-weighted imaging data. Endocardial and epicardial borders were manually delineated according to the reconstructed diffusion-weighted data. Both region of interest–based analysis and segmental analysis were performed as follows. Regions of interest (ROI) manually drawn in accordance with standards set by the European Association for Cardiovascular Imaging (8) for the analysis of MD and FA. After dividing each section into six equiangular segments starting from the anterior interventricular junction (9), segmental E2A averages were derived. Statistical analyses were performed using IBM SPSS Statistics software (version 26.0). The continuous variables were reported as means ± standard deviations. Comparison was performed using an independent-sample parametric statistical test. Statistical significance was accepted when a P-value was <0.05 (two-tailed test).Result
cDTI acquisition was successful scanned in 7 pigs with CMD and 3 normal pigs ((18.7 ± 1.1 vs. 18.8 ± 1.4 kg, P = 0.877). Results showed that pigs with CMD had higher MD, lower FA, and lower E2A compared with normal pigs (mean MDnormal = 1.50 ± 0.02, mean MDCMD = 1.65 ± 0.02, P = 0.001; mean FAnormal = 0.35 ± 0.01, mean FACMD = 0.27 ± 0.01, P < 0.001; mean E2Anormal = 53.33° ± 0.88, mean E2ACMD = 46.57° ± 1, P = 0.004) (Table 1).Discussion
cDTI were used for evaluating the changes of myocardial microenvironment for pig models with CMD when compared with healthy models. This study showed that pigs with CMD had higher MD, lower FA, and lower E2A, which convinced that DTI technology can be used as a predictor for CMD. In human studies of myocardial infarct, increased MD and decreased FA in the infarcted area have been found (4-6). Histological verification that the changes were associated with cardiomyocyte swelling and necrosis, extracellular matrix expansion, and to a lesser extent, collagen fibrillogenesis. In addition, E2A decreased in the infarcted area by DTI, suggesting that disruption of subendocardial cardiomyocyte structure affects the ability of the myometrium to reorient during systole (6). Our study found that similar changes in myocardial infarct happened in CMD pigs, which might investigate that above pathological changes also existed during the progression of CMD, which need more models rather than single-center to further verify.Conclusion
Diffuse tensor imaging can be used as a predictor for pig models of CMD, which could provide new insight into the assessment of treatment and diagnosis.Acknowledgements
No acknowledgement found.References
1. Nielles-Vallespin S, Khalique Z, Ferreira PF et al. Assessment of Myocardial Microstructural Dynamics by In Vivo Diffusion Tensor Cardiac Magnetic Resonance. Journal of the American College of Cardiology 2017;69:661-676.
2. Teh I, Romero RW, Boyle J et al. Validation of cardiac diffusion tensor imaging sequences: A multicentre test-retest phantom study. NMR Biomed 2022;35:e4685.
3. Holmes AA, Scollan DF, Winslow RL. Direct histological validation of diffusion tensor MRI in formaldehyde-fixed myocardium. Magnetic Resonance in Medicine 2000;44:157-161.
4. Ariga R, Tunnicliffe EM, Manohar SG et al. Identification of Myocardial Disarray in Patients With Hypertrophic Cardiomyopathy and Ventricular Arrhythmias. J Am Coll Cardiol 2019;73:2493-2502. 5. Moulin K, Viallon M, Romero W et al. MRI of Reperfused Acute Myocardial Infarction Edema: ADC Quantification versus T1 and T2 Mapping. Radiology 2020;295:542-549.
6. Wu MT, Su MY, Huang YL et al. Sequential changes of myocardial microstructure in patients postmyocardial infarction by diffusion-tensor cardiac MR: correlation with left ventricular structure and function. Circ Cardiovasc Imaging 2009;2:32-40, 6 p following 40.
7. Stoeck CT, von Deuster C, Genet M, Atkinson D, Kozerke S. Second-order motion-compensated spin echo diffusion tensor imaging of the human heart. Magnetic Resonance in Medicine 2016;75:1669-1676.
8. Messroghli DR, Moon JC, Ferreira VM et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson 2017;19:75.
9. Cerqueira MD, Weissman NJ, Dilsizian V et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105:539-42.