1521
3D Isotropic black-blood cine MRI of intracranial arteries1Radiology, University of Washington, Seattle, WA, United States, 2Bioengineering, University of Washington, Seattle, WA, United States, 3Surgery, University of Washington, Seattle, WA, United States, 4University of Utah, Salt Lake City, UT, United States
Synopsis
Keywords: Vessels, Data Acquisition
Pulsatile motion of intracranial arteries may provide useful diagnostic information of intracranial vascular pathology. We develop an isotropic 3D black-blood whole brain MRI method for assessment of intracranial vascular pulsation. Comparison of the black-blood with 2D bright-blood PC-MRA and assessment of luminal boundary changes across the cardiac cycle suggest that pulsation of large intracranial arteries can be detected and measured by black-blood cine MRI.Introduction
Pulsation of the vasculature may provide information about the pathological conditions of the vasculature. Dynamic MRI of vascular pulsation with each cardiac cycle may thus provide new means of studying disease processes in multiple disorders such as atherosclerosis, aneurysms, dissection etc. Bright blood 2D cine MRI has previously been demonstrated to provide such diagnostic information in aortic pathologies [1]. Black-blood cine MRI has also been demonstrated for use in carotid arteries [2]. However, detection and measurement of pulsation of intracranial arteries is challenging and has not been evaluated before. In contrast to relatively straight arteries such as aorta/carotids, intracranial arteries have complex geometry, branching structure and are distributed over the brain. Hence cine MRI of intracranial arteries requires 3D isotropic coverage of the whole brain such that different intracranial arteries may be reformatted in their axial sections for evaluation. Moreover, bright blood cine MRI does not work for intracranial arteries since there is surrounding CSF with similar signal appearance. Therefore, in this work, we develop a 3D isotropic black-blood cine MRI for intracranial vascular application.Methods
Scans were performed on a Philips Ingenia whole body 3T scanner. Subjects were fitted with a 32-channel head coil. Peripheral pulse gating was applied and used for cardiac triggering. A segmented 3D gradient echo acquisition was applied after a twice-refocused motion-sensitized driven equilibrium (MSDE) black-blood pre-pulse [3] followed by spectral inversion recovery and saturation pulse for fat suppression [4] with each segment. Ten segments corresponding to ten cardiac phases were obtained every heartbeat. Images corresponding to the ten cardiac phases were reconstructed using compressed sensing and retrospective binning into cardiac phases. MSDE parameters were optimized such that the shortest pre-pulse with effective blood suppression was obtained. Images were obtained with a resolution of 1x1x1mm (interpolated to 0.5x0.5x0.5mm) with AP/FH/RL field-of-view of 20x20x16cm and GRE TR/TE of 10/4ms, flip angle 6 degrees. Eighteen k-space lines were obtained with centric ordering per segment. Compressed sensing acceleration of 8x was applied with a total scan time of 5 minutes. A 2D single slice bright blood phase-contrast cine MRA was also obtained for comparison at the level of the basilar artery (center of basilar artery segment). Imaging parameters were as follows: AP/RL Field-of-view of 20x16cm, 1x1mm (interpolated to 0.5x0.5mm) in-plane resolution, 5 mm axial slice thickness, TR/TE 10/6 ms, flip angle 10 degrees, FH velocity encoding 90cm/s, 2-minute scan time.Results
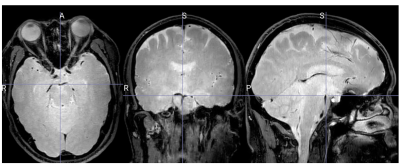
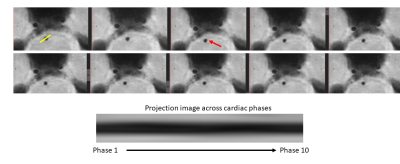
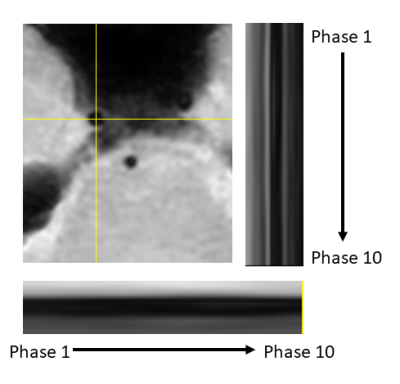
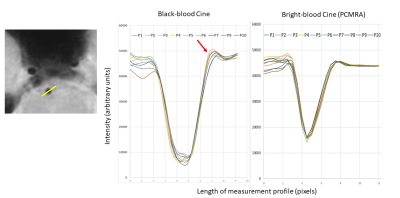
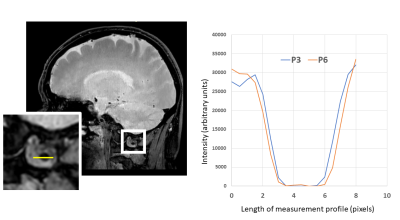
Three volunteers were scanned according to institutional IRB guidelines. All scans were successful with good depiction of intracranial vasculature with whole brain coverage (figure 1). Large arteries showed change in vessel caliber across the cardiac cycle (basilar artery shown in figure 2). Projections across the cardiac cycle were obtained at different vessel segments to examine the feasibility of detecting intracranial vascular pulsation (figure 2 and 3). Luminal boundary changes were visible in cross-sectional planes and projection images. However, there was also complex motion in three-dimensions with artery motion side to side suggesting that full 3D measurement is necessary to characterize the pulsation accurately. Both black-blood cine and bright blood cine (PC-MRA) showed similar signal profiles when line profiles were obtained at vessel boundaries (figure 4) showing that black-blood cine can obtain similar information as 2D PC-MRA along with whole brain 3D isotropic coverage. Moreover, vessel luminal boundary movements were more visible on black-blood MRI (figure 4) indicating the feasibility for measurement. Clear differences in luminal boundary (expansion and contraction) were visible corresponding to the systole/diastole of the cardiac cycle (figure 5).Conclusions
An isotropic 3D black-blood cine MRI sequence for intracranial vasculature pulsation assessment was developed with a short scan time. Luminal boundary changes across the cardiac cycle were appreciable and corresponded with expected changes during systole/diastole and with 2D bright blood cine MRI. The pulsation was visible and detectable by line profile assessment of luminal boundaries suggesting feasibility for pulsation measurement. Further need for 3D measurement methods is indicated based on the complex motion assessed using our black-blood cine MRI sequence.Acknowledgements
No acknowledgement found.References
1] Vos AW, Wisselink W, Marcus JT, Manoliu RA, Rauwerda JA. Aortic aneurysm pulsatile wall motion imaged by cine MRI: a tool to evaluate efficacy of endovascular aneurysm repair? Eur J Vasc Endovasc Surg. 2002 Feb;23(2):158-61. doi: 10.1053/ejvs.2001.1558. PMID: 11863334.
[2] Koktzoglou I. 4D Dark blood arterial wall magnetic resonance imaging: methodology and demonstration in the carotid arteries. Magn Reson Med. 2013 Apr;69(4):956-65. doi: 10.1002/mrm.24647. Epub 2013 Feb 11. PMID: 23400824.
[3] Wang J, Yarnykh VL, Yuan C. Enhanced image quality in black-blood MRI using the improved motion-sensitized driven-equilibrium (iMSDE) sequence. J Magn Reson Imaging. 2010 May;31(5):1256-63. doi: 10.1002/jmri.22149. PMID: 20432365; PMCID: PMC2908521.
[4] Balu N, Yarnykh VL, Chu B, Wang J, Hatsukami T, Yuan C. Carotid plaque assessment using fast 3D isotropic resolution black-blood MRI. Magn Reson Med. 2011 Mar;65(3):627-37. doi: 10.1002/mrm.22642. Epub 2010 Oct 12. PMID: 20941742; PMCID: PMC3042490.
Figures