1519
Diffusion Weighted-Viscosity Imaging for Atherosclerotic Plaques
Mayuka Seguchi1, Yuki Kanazawa1, Tosiaki Miyati2, Masafumi Harada1, Mitsuharu Miyoshi3, Yuki Matsumoto1, Hiroaki Hayashi2, Yasuhisa Kanematsu1, and Yasushi Takagi1
1Tokushima University, Tokushima, Japan, 2Kanazawa University, Kanazawa, Japan, 3GE Healthcare Japan, Hino, Japan
1Tokushima University, Tokushima, Japan, 2Kanazawa University, Kanazawa, Japan, 3GE Healthcare Japan, Hino, Japan
Synopsis
Keywords: Atherosclerosis, Diffusion/other diffusion imaging techniques
We developed a Dw-viscosity imaging method to identify atherosclerotic plaques. First, we conducted experiments to determine ADC and viscosity changes of four different concentrations of glycerin solution, and determined the viscosity model. Second, a clinical study was carried out, and Dw-viscosity imaging was performed. As a result, we found that there was a significant difference between with and without hemorrhage findings (P < 0.01). Dw-viscosity imaging enables us to obtain more detailed information about atherosclerotic plaques such as intraplaque hemorrhage.INTRODUCTION
Early treatment of plaque rapture is necessary because it becomes a high risk for causing a stroke. High-risk factors related to atherosclerotic plaque rapture are calcification, hemorrhage, and fibrosis. Multi-contrast MR images, such as T1-weighted (T1w) and T2-weighted (T2w), and MRA, are available when using MRI in diagnosis. However, distinguishing plaque structures is difficult during clinical studies, because the signals related to atherosclerotic plaques from conventional MRI using T1w and T2w images are usually superimposed. Recently, there have been reports concerning plaque imaging using diffusion-weighted (Dw) -MRI. T1 and apparent diffusion coefficient (ADC) values can be used to distinguish the lipid-rich necrotic core (LRNC), fibrosis, and hemorrhage [1, 2]. ADC depends on viscosity as well as limitations due to physical structures. Viable cell density is the main biological parameter responsible for restricted diffusion [3]. If we determine the viscosity of an atherosclerotic plaque, it may be possible to prevent plaque rupture. The purpose of our study was to develop a Dw-viscosity imaging method to determine the contents of atherosclerotic plaques.METHODS
Figure 1 shows a schematic illustration of Dw-viscosity imaging of an atherosclerotic plaque. First, we conducted experiments to determine ADC and viscosity changes of four different concentrations of glycerin solution (50, 60, 70, and 80 wt%). These samples were heated in a water bath at 40 C˚ for 10 min, and then the viscosity of each sample was measured. Dw dataset of multi-b-values was acquired at a constant temperature of 40 C˚ using a case composed of rigid urethane foam. The case makes it possible to maintain a steady state temperature for 20 min. On a 3 Tesla MR scanner, Dw-MRI was performed with single-shot echo-planner imaging (SSEPI) of Field-of-view Optimized and Constrained Undistorted Single-shot (FOCUS). Dw-MR imaging parameters were as follows; repetition time (TR), 3000 ms; echo time (TE), 58.1 ms; b-values, 0-1500 s/mm2 (15 points). The ADC of each glycerin sample was calculated using a mono-exponential decay model. The relationship between measured viscosity and ADC value was determined by curve fitting.Second, a clinical study was carried out. This prospective study was approved by the institutional review board, and all imaging datasets for 18 patients with carotid stenosis were acquired after informed consent was obtained. After the MRI study, all patients had carotid endarterectomy (CEA). Dw-MRI was performed using three-point b-values (0, 250, and 500 s/mm2) SSEPI of FOCUS. The same Dw-MR imaging parameters used in the above phantom study were applied. T1w and T2w images using three-dimensional Fast Spin-Echo with Motion-Sensitized Driven Equilibrium (3D-FSE-MSDE) were acquired. The T1w- and T2w- 3D-FSE-MSDE imaging parameters were TR/TE = 500 ms/26.5 ms, 2500 ms/62.3 ms. Then, regions of interest were manually outlined on MR images for areas which contain atherosclerotic plaques and sternocleidomastoid muscle. Based on the ADC values calculated from each b-value imaging dataset in the atherosclerotic plaque areas, a Dw-viscosity image was generated using the model derived from the above glycerin experiments. T1w- and T2w-signal-ratios were defined as each plaque signal intensity and normalized with muscle. Mann-Whitney U test was performed.
RESULTS & DISCUSSION
Table 1 shows the relationship between glycerin concentrations and measured viscosities at 40 C˚. Figure 2 shows the relationship between viscosity and ADC for glycerin at 40 C˚. There were strong correlations with exponential approximation (R2 = 0.95). Figure 3 shows the relationship of measured Dw-viscosity for atherosclerotic plaques with and without hemorrhage findings. There is a significant difference between with and without hemorrhage findings (P < 0.01). Figure 4 shows T1w images, T2w images, ADC maps, and Dw-viscosity images of each representative subject with atherosclerotic plaques. The mean viscosity values of upper and lower subjects were 1.62 and 0.42 mPa·s. In our study, although the viscosity of the plaque was converted from that of glycerin, we need to consider the viscosity including not only self-diffusion but also restricted diffusion between complex structures and/or cells within the plaque. As a technical aspect, although Dw-image could be influenced by the heartbeat's synchronized wall motion, the influence of the heartbeat was averaged and reduced by the multiple numbers of excitations.CONCLUSION
Dw-viscosity imaging makes it possible to derive more detailed information about atherosclerotic plaques such as intraplaque hemorrhage. This information may be used as a risk index, e.g., plaque rupture.Acknowledgements
No acknowledgement found.References
- Ota H, Tamura H, Itabashi R, et al. Quantitative characterization of carotid plaque components using MR apparent diffusion coefficients and longitudinal relaxation rates at 3T: A comparison with histology. J Magn Reson Imaging. 2018;48(6):1657-1667.
- Yao B, Yang L, Wang G, et al. Diffusion measurement of intraplaque hemorrhage and intramural hematoma using diffusion weighted MRI at 3T in cervical artery. Eur Radiol. 2016;26(10):3737-43.
- Mishra AM, Gupta RK, et al. Biological correlates of diffusivity in brain abscess. Magn Reson Med. 2005;54(4):878-85.
Figures
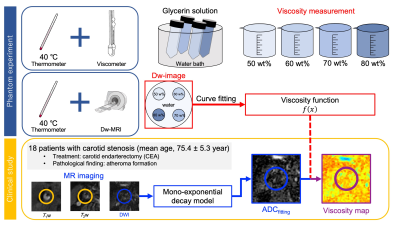
Figure 1 A schematic illustration of Dw-viscosity imaging of an atherosclerotic plaque.
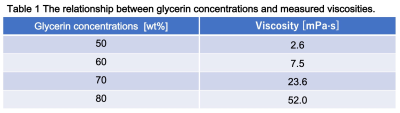
Table 1 The relationship between glycerin concentrations and measured viscosities.
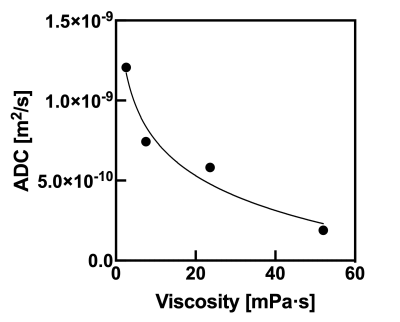
Figure 2 The relationship between viscosity and ADC for glycerin at 40 C˚.
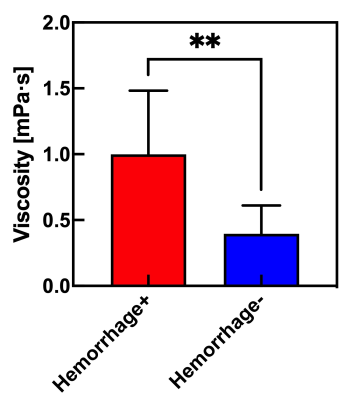
Figure 3 The relationship of measured Dw-viscosity for atherosclerotic plaques with and without hemorrhage findings.
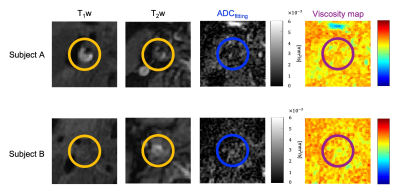
Figure 4 MR images for two representative subjects with atherosclerotic plaques. Left to right, T1w images, T2w images, ADC maps, and Dw-viscosity images. Upper is a 78-year-old man, lower is a 78-year-old man.
DOI: https://doi.org/10.58530/2023/1519