1518
Multifunctional Nanoparticles for Detection and Treatment of Atherosclerotic Vulnerable Plaques
Man Ye1
1Renmin Hospital of Wuhan University, Wuhan, China
1Renmin Hospital of Wuhan University, Wuhan, China
Synopsis
Keywords: Atherosclerosis, Molecular Imaging
The instability of atherosclerotic plaque is seriously harmful to human health. The nanoparticle molecular probe we prepared can simultaneously achieve magnetic resonance and ultrasound imaging, which is expected to reflect the pathophysiological characteristics of plaque at the molecular level. The phase change induced by low intensity focused ultrasound (LIFU) can cause the apoptosis of macrophages in plaque,which has the potential for phase change ablation of plaque.1 It provides a simple, effective, safe and non-invasive new method for early diagnosis, treatment and efficacy monitoring of vulnerable plaque.Background or purpose
Atherosclerosis is an important pathological basis for the occurrence and development of cardiovascular diseases. Vulnerable atherosclerotic plaques are prone to sudden rupture, leading to fatal events.2 In this study, we prepare a highly efficient, sensitive and specific multi-modal nanoparticle integrating diagnosis and treatment, for realizing the in vivo and non-invasive early identification of atherosclerotic vulnerable plaque, and early treatment of atherosclerotic vulnerable plaque at the molecular level.Methods
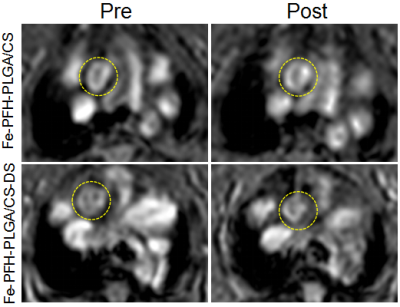
Multifunctional Fe-PFH-PLGA/CS-DS NPs were constructed by improved double emulsification method and electrostatic adsorption method.3 The physicochemical properties of the NPs were characterized by dynamic light scattering detector, transmission electron microscopy, atomic absorption spectrometer, fourier transform infrared spectroscopy, confocal laser scanning microscope and flow cytometry analysis. Magnetic resonance and ultrasound imaging were performed to analyze the imaging properties of the NPs. RAW264.7 was extracted and cultured from mouse macrophages. The targeting ability of NPs to macrophages in vitro was preliminarily evaluated. The cytotoxicity of RAW264.7 was analyzed by CCK-8 method. To establish apolipoprotein E (ApoE) knockout (KO) (apoE-/-) mice model of atherosclerotic vulnerable plaque, the expression of SR-A receptor in vulnerable plaque was analyzed by pathological and immunohistochemical methods, and to explore the feasibility and accuracy of targeting diagnosis of atherosclerotic vulnerable plaque. To investigate the potential of NPs combined with LIFU for phase change ablation of plaque in vitro and in vivo.Results
The Fe-PFH-PLGA/CS-DS NPs were fabricated successfully, with the ability to undergo phase transition by LIFU irradiation to achieve US imaging; a high carrier rate of Fe3O4 had a good negative enhancement effect on magnetic resonance imaging. The NPs had a high binding affinity for activated macrophages and could be endocytosed by the macrophages and notably induced apoptosis under LIFU irradiation by an acoustic droplet vaporization (ADV) effect in vitro. Furthermore, in an ex vivo atherosclerotic plaque model of apoE -/- mice induced by high cholesterol, the NPs selectively accumulated at the sites of SR-A expressed on the activated macrophages of the aortic region. This result was also confirmed by MRI in vivo, where the NPs could be targeted to the aortic plaque and reduced the T2* signal. The LIFU-induced phase transition could lead to the apoptosis of macrophages on plaques in vivo.Discussion and conclusions
In this study, PLGA was used as a carrier to prepare NPs by a double emulsification method. The oleic acid-modified Fe3O4 was embedded in the shell membrane of the NPs, PFH was encapsulated in the nuclei of the NPs, and DS was electrostatically adsorbed onto the NPs using CS without involving harsh synthesis steps. This process specifically targets the SR-A of macrophages, thus enabling the diagnosis of atherosclerotic plaques and evaluation of plaque vulnerability followed by phase transition inside macrophages to damage the macrophages at the cellular level. To sum up, the Fe-PFH-PLGA/CS-DS NPs may be applied as multimodal and multifunctional probes and are expected to enable the specific diagnosis and targeted therapy of vulnerable plaques.Acknowledgements
No acknowledgement found.References
1. Hou J, Zhou J, Chang M, et al. LIFU-responsive nanomedicine enables acoustic droplet vaporization-induced apoptosis of macrophages for stabilizing vulnerable atherosclerotic plaques. Bioact Mater. 2022; 16:120-133.
2. Oikonomou E, Leopoulou M, Theofilis P, et al. A link between inflammation and thrombosis in atherosclerotic cardiovascular diseases: Clinical and therapeutic implications. Atherosclerosis. 2020; 309:16-26.
3. Yao Y, Li B, Yin C, et al. A Folate-Conjugated Dual-Modal Fluorescent Magnetic Resonance Imaging Contrast Agent that Targets Activated Macrophages In Vitro and In Vivo. J Biomed Nanotechnol. 2016;12(12):2161-2171.
DOI: https://doi.org/10.58530/2023/1518