1512
Feasibility of Measuring Magnetic Resonance Elastography-derived Stiffness in Aortic Dissection
Adnan A. Hirad1, Faisal fakhouri2, Brian Raterman3, Dakota Gonring1, Arunark Kolipaka3, and Doran Mix1
1University of Rochester, Rochester, NY, United States, 2King Saud University, Riyadh, Saudi Arabia, 3The University of Ohio, Columbus, OH, United States
1University of Rochester, Rochester, NY, United States, 2King Saud University, Riyadh, Saudi Arabia, 3The University of Ohio, Columbus, OH, United States
Synopsis
Keywords: Vessel Wall, Blood vessels
Type-B aortic dissection represents a serious medical emergency with up to a 50% associated 5-year mortality caused by thoracic aorta, dissection-associated aneurysmal (DAA) degeneration, and rupture. Unfortunately, conventional diagnostic methods cannot distinguish high-risk DAAs that benefit from surgical intervention from stable DAAs. Our goal is to use DAA stiffness measured with magnetic resonance elastography (MRE) as a biomarker to distinguish high-risk DAAs from stable DAAs. This is a feasibility study using MRE to 1) measure stiffness in hydrogel phantoms with human-like dissection geometries and 2) demonstrate the first successful application of MRE to the thoracic aorta of a human volunteer.Introduction
Type-B Aortic Dissection (TBAD) represents a serious medical emergency with up to a 50% associated 5-year mortality (1, 2). Dissection-associated aneurysmal (DAA) degeneration and rupture cause long-term TBAD mortality, and some clinicians have advocated for early surgical intervention. A significant gap in research exists wherein clinicians cannot separate patients with DAA from those with stable dissection. Fundamentally, the aneurysmal dilation of the aorta derives from a mismatch between its wall properties and pulsatile stresses (3). This question can be resolved by understanding the natural history of material properties of the dissected aortic wall and its interaction with dissection-specific hemodynamics. These findings would likely aid in developing predictive indices of this degenerative process and provide better guidance for patient-specific risks and benefits of surgical intervention. To address this gap, a few challenges should be addressed: 1) experimental models to study the disease and 2) clinical imaging modalities that offer information beyond aortic morphometry. A complex hydrogel model of TBAD with programmatically controlled geometries and material properties was developed to overcome the first challenge. To overcome the second challenge, magnetic resonance elastography (MRE) will be utilized to elucidate noninvasively vascular mechanical properties in vivo. This study aims to develop indices of DAA material properties by implementing and validating MRE on hydrogel phantoms with human-like dissection geometries. Then the developed MRI techniques were tested on a healthy volunteer and in future TBAD patients.Methods
Phantom Fabrication:A mold consistent with human TBAD geometries was created using a 3D fused deposition modeling (FDM) printer. Different polyvinyl alcohol cryogel (PVA-c) concentrations were injected into the molds resulting in four phantoms with two differing sidewall stiffness (Figure 1). Four TBAD phantoms were generated, each comprised of heterogeneous material properties consisting of two differing PVA-c concentrations that mimic increasing stiffness throughout a range of human aortic tissue (one side was always 10% PVA-c and the other side was either 10%, 15%, 20%, or 25% PVA-c.)
Phantom MRE Scan & Image Processing:
TBAD phantoms were scanned in a 3T-MR scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany). Mechanical vibration was introduced into both sides of the phantom using a Resoundant driver system ((Resoundant, Rochester, MN, USA). As shown in Figure 1, the passive driver was 3D printed to serve as a holder for the phantom and a mechanical actuator at the same time. A cardiac-gated spin-echo echo planar imaging (SE-EPI) sequence was used with the following parameters: 5mm thick coronal slices, FOV of 32x32cm2, acquisition matrix of 256x256, TR of 228.48ms, TE of 11.93ms, motion encoding gradients (MEG) frequency of 100Hz, and mechanical frequency of 70Hz.
Due to the simplicity of the phantom structure and the presence of well-distinguished planar waves, shear stiffness was manually calculated from the wavelength of a planar propagating wave using the equation μ = ρf2λ2, where μ is the shear stiffness, ρ is tissue density, f is the external mechanical vibration frequency, and λ is the propagating wave wavelength.
Healthy Volunteer MRE Scan & Image Processing:
A thoracic aorta of a healthy volunteer was scanned in the same 3T-MR scanner MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany). Mechanical vibration was introduced into the thoracic aorta by placing a commercially available passive driver (Resoundant, Rochester, MN, USA) under the volunteer's left shoulder’s scapular bone in the supine position, as shown in Figure 2. The same EPI sequence was used with the following protocol parameters: 6mm sagittal slices, FOV of 36x36cm2, acquisition matrix of 256x256, TR of 171.36ms, TE of 11.88ms, and 8 cardiac segments. Similar to the phantom scans, the MEG frequency was 100Hz, and the mechanical frequency was 70Hz.
Due to the complexity of the propagating wave within the human body and the difficulty of getting clear planar waves, shear stiffness was calculated by using the local frequency estimation (LFE) algorithm (MRElab, Mayo Clinic, Rochester, MN) with a directionally filtered bandpass filter with cutoff values of 1-40 waves/FOV. To minimize blood flow artifacts, end-diastolic images were used.
Results
Phantom MRE:Figure 3 shows MRE magnitude and wave images of both sides of TBAD phantoms. The MRE shear stiffness of the 10 % side of all the TBAD phantoms was estimated to be 32.19±2.66kPa. The MRE shear stiffness of the 15%, 20%, or 25% sides of the TBAD phantoms was estimated at 51.48 kPa, 59.29 kPa, and 73.53 kPa, respectively. Figure 4 shows the correlation (R2 = 0.99) between MRE and rheometric stiffness measurements of the four phantoms with different PVA-c concentrations.
Volunteer MRE:
Figure 5 shows examples of magnitude images, wave images, and stiffness maps of the thoracic aorta of a healthy volunteer. The mean shear stiffness of the thoracic aorta was 3.37±0.83kPa.
Discussion and Conclusion
TBAD phantoms with different stiffnesses were studied, and a healthy volunteer thoracic aorta was scanned. We were able to show that MRE generated stiffness of the TBAD phantoms highly correlated with rheometry-measured stiffness. We also demonstrated the feasibility of scanning the thoracic aorta of a human subject, which is known to be challenging due to blood flow and motion artifacts.Acknowledgements
No acknowledgement found.References
1. M. M. Sigman, O. P. Palmer, S. W. Ham, M. Cunningham, F. A. Weaver, Aortic morphologic findings after thoracic endovascular aortic repair for type B aortic dissection. JAMA Surg 149, 977-983 (2014).
2. I. Sultan et al., Predicting Distal Aortic Remodeling After Endovascular Repair for Chronic DeBakey III Aortic Dissection. Ann Thorac Surg 105, 1691-1696 (2018).
3. E. K. Prokop, R. F. Palmer, M. W. Wheat, Jr., Hydrodynamic forces in dissecting aneurysms. In-vitro studies in a Tygon model and in dog aortas. Circ Res 27, 121-127 (1970).
Figures

Figure 1: (top left) Shells A, B, and C, disassembled (top middle) Shells A, B, and C, assembled into the mold (top right) Cyrogel phantom removed from mold (bottom left) in house designed and built passive driver, with dual diaphragms to deliver vibrations to each side of the phantom separately but simultaneously.
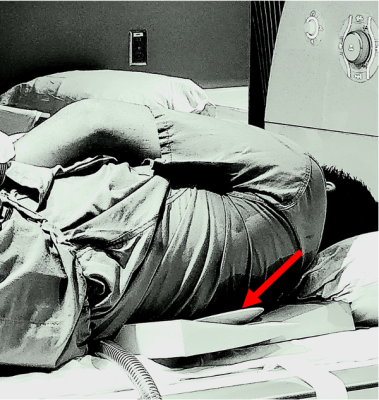
Figure 2: Healthy volunteer setup in which the passive driver (red arrow) is under the scapular bone and the left lateral edge of the vertebral column.
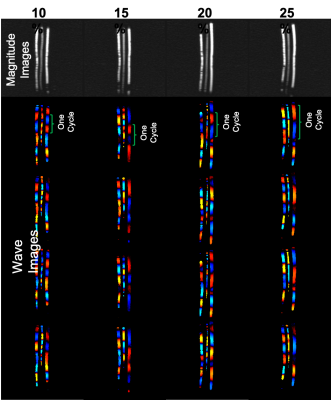
Figure 3: MRE magnitude and wave images of each side of the TBAD phantoms with PVA-c concentrations of 10%, 15%, 20%, and 25%. As shown by the green curled brackets, the wavelength for a given cycle increases with increasing stiffness (i.e. increasing PVA-c concentration).
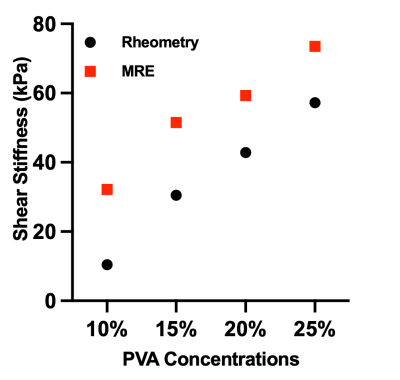
Figure 4: Correlation plot (R2=0.99) between MRE and rheometric stiffness measurements of phantoms with different PVA-c concentrations of 10%, 15%, 20, and 25%.
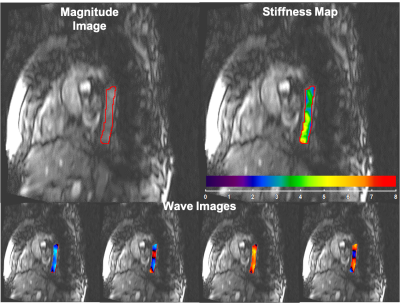
Figure 5: Magnitude image, four snap shots of wave images at different time points in read direction, and stiffness map of the thoracic aorta of a healthy volunteer with a mean shear stiffness of 3.37±0.83kPa.
DOI: https://doi.org/10.58530/2023/1512