1509
Deep Learning-Based Automatic Segmentation of Arterial Plaques in MR Vessel Wall Images
Long Yang1,2, Xiong Yang3, Zhenhuan Gong3, Yufei Mao3, Guanxun Cheng4, Ke Wu3, Cheng Li3, Ye Li1, Dong Liang1, Xin Liu1, Hairong Zheng1, Zhanli Hu1, and Na Zhang1
1Lauterbur Research Center for Biomedical Imaging, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 2School of Computer Sciences, University Sains Malaysia, Penang, Malaysia, 3Department of Image Advanced Analysis of HSW BU, Shanghai United Imaging Healthcare Co., Shanghai, China, 4Department of Radiology, Peking University Shenzhen Hospital, Shenzhen, China
1Lauterbur Research Center for Biomedical Imaging, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 2School of Computer Sciences, University Sains Malaysia, Penang, Malaysia, 3Department of Image Advanced Analysis of HSW BU, Shanghai United Imaging Healthcare Co., Shanghai, China, 4Department of Radiology, Peking University Shenzhen Hospital, Shenzhen, China
Synopsis
Keywords: Vessel Wall, Atherosclerosis, atherosclerotic plaque, morphological quantitative assessment
Manual segmentation of atherosclerotic plaque for quantitative assessment is a time-consuming process. In this study, a convolutional neural network based automatic segmentation method named Vessel-Segnet was proposed for quantitative evaluation of lumen, vessel wall and plaque based on MR vessel wall images. The proposed method achieved the best segmentation performance with the highest dice similarity coefficient and the lowest average surface distance among six models. In terms of morphological quantitative evaluation, the proposed method achieved excellent agreement with manual method. Overall, the proposed method can quickly and accurately realize the segmentation of lumen, vessel wall and plaque for quantitative evaluation.INTRODUCTION
Stroke is the second leading cause of death worldwide1. Assessing plaque progression and stability is critical for ischemic stroke prevention, monitoring, and treatment2,3. High-resolution magnetic resonance vessel wall imaging (MRVWI) is an important and promising method for evaluating plaque4,5. Researchers can qualitatively evaluate plaque according to MRVWI to assess risks, monitor plaque progression and evaluate therapeutic effects6,7. however, manual plaque identification is difficult and time-consuming in large-scale slices and requires experienced professional radiologists. In this study, an CNN-based automated method was developed for lumen, vessel wall and plaque segmentation based on MR vessel wall images and evaluated on a large number of patients with ischemic stroke.METHODS
All MR vessel wall images were acquired from 146 patients on 3T whole-body MR system (uMR780, United Imaging Healthcare Shanghai, China). A total of 209 plaques were detected and 2D slices of these plaques were reconstructed from the acquired 3D MRVWI to delineate the vessel walls and plaques by 4 radiologists with more than 5 years of experience. Among them, 1517 and 411 2D slices of 159 plaques were used for training and validation to construct the models. A total of 547 slices of the remaining 50 plaques were used as independent test sets to evaluate the developed models. Before image inputting, interpolation, normalization and histogram equalization are performed. The final input image is 2 * 400 * 400 (channel * width * height, for plaque task, 1st and 2nd channel is image and prior knowledge - vessel wall mask, respectively) and 1 * 400 * 400 (for lumen and vessel wall task). A deep learning architecture named Vessel-Segnet was constructed for segmentation tasks which refers to the structures of U-Net8 and SegNet9 and adopts several effective configurations. The main architecture of the network is shown in Figure 1. To more clearly show the contribution of each model unit and the effects of reasonable combinations of different units, a series of ablation experiments were carried out in this study. The dice similarity coefficient (DSC) and average surface distance (ASD) were used to evaluate the similarity of the segmentation results between the manual and automatic methods. The intraclass correlation coefficient (ICC) and Bland Altman plots were used to evaluate the agreement between the quantitative results of the manual and automatic methods.RESULTS
In the test set, the DSC and ASD obtained by the proposed method achieved the best among all models: the average DSC and ASD of lumen, vessel wall, plaque (with prior) and plaque (without prior) reach 97.88% ± 5.00%, 92.39% ± 5.47%, 85.06% ± 10.87%, 74.47 ± 15.98% and 0.048 ± 0.088mm, 0.117± 0.115mm, 0.223± 0.255mm, 0.419± 0.388mm, respectively. The ICC values of lumen area, vessel wall area, plaque area (with prior), plaque area (without prior) and plaque burden calculated by the proposed method compared with that obtained by the manual method reached 0.985, 0.92, 0.941, 0.810 and 0.960, respectively. The DSC, ASD and ICC values obtained by different models are summarized in figure 2. Figure 3 shows representative lumen, vessel wall and plaque segmentation results obtained by each model. Among the results, the segmentation result of Vessel-Segnet is the closest to that of ground truth. The Bland‒Altman plots of lumen area, vessel wall area, plaque area and plaque burden obtained by the proposed automatic segmentation method and manual segmentation method were shown in Figure 4. The mean differences in the lumen area, vessel wall area, plaque area and plaque burden between the two methods were -0.1 mm², 2.3 mm², -0.7 mm² and -0.021, respectively. Most of the points were within 1.96 times the standard deviation, which shows good agreement. The results of the ablation experiments are shown in Figure 5. When the residual unit, CBAM, stride convolutions and PReLU were all applied, the DSC score showed the greatest improvement of 3.1%. When the CCQ structure was adopted, the convolution kernel was increased, or both operations were performed, the DSC value improved by 2%, 3.3% and 4.2%.DISCUSSION
This study proposed and evaluated a CNN-based automatic segmentation method for quantitative evaluation of lumen, vessel wall and plaque based on MRVWI. The proposed method not only got high similarity with the manual method in segmentation of lumen, vessel wall and plaque, but also got excellent agreement with the manual method in the morphological quantitative evaluation. Moreover, the use of prior knowledge further improves the performance (>10% DSC) of plaque segmentation. Compared with other 5 popular methods, the proposed method achieved the best segmentation performance with the highest dice similarity coefficient and the lowest average surface distance. The reasonable design of the structure makes the proposed model achieve the best segmentation results. Thus, the proposed method can be potentiality replaced manual method and applied to evaluate plaque progression and treatment effects and ultimately improve the prevention, monitoring and treatment of patients at risk of ischemic stroke.CONCLUSION
The proposed CNN-based segmentation method can quickly and accurately segment arterial lumen, vessel wall and plaque for morphological quantitative evaluation in MRVWI. Among the six automatic methods, the proposed method is the best approach.Acknowledgements
The study was partially supported by the National Natural Science Foundation of China (81830056), the Key Laboratory for Magnetic Resonance and Multimodality Imaging of Guangdong Province (2020B1212060051), Shenzhen Basic Research Program (KCXFZ202002011010360), the Guangdong Innovation Platform of Translational Research for Cerebrovascular Diseases of China, and the Key Technology and Equipment R&D Program of Major Science and Technology Infrastructure of Shenzhen (202100102 and 202100104).References
1. Organization WH. Global Health Estimates: Life expectancy and leading causes of death and disability. 2020 Date.2. Gorelick PB, Sacco RL, Smith DB, Alberts M, Mustone-Alexander L, Rader D, Ross JL, Raps E, Ozer MN, Brass LM. Prevention of a first stroke: a review of guidelines and a multidisciplinary consensus statement from the National Stroke Association. Jama. 1999; 281:1112-1120.
3. Madden JA. Role of the vascular endothelium and plaque in acute ischemic stroke. Neurology. 2012; 79:S58-S62.
4. Saba L, Yuan C, Hatsukami T, Balu N, Qiao Y, DeMarco J, Saam T, Moody A, Li D, Matouk C. Carotid artery wall imaging: perspective and guidelines from the ASNR vessel wall imaging study group and expert consensus recommendations of the American Society of Neuroradiology. Am J Neuroradiol. 2018; 39:E9-E31.
5. Mandell D, Mossa-Basha M, Qiao Y, Hess C, Hui F, Matouk C, Johnson M, Daemen M, Vossough A, Edjlali M. Intracranial vessel wall MRI: principles and expert consensus recommendations of the American Society of Neuroradiology. Am J Neuroradiol. 2017; 38:218-229.
6. Gupta A, Baradaran H, Schweitzer AD, Kamel H, Pandya A, Delgado D, Dunning A, Mushlin AI, Sanelli PC. Carotid plaque MRI and stroke risk: a systematic review and meta-analysis. Stroke. 2013; 44:3071-3077. doi:10.1161/STROKEAHA.113.002551. PMID: 23988640.
7. Yuan C, Kerwin WS, Yarnykh VL, Cai J, Saam T, Chu B, Takaya N, Ferguson MS, Underhill H, Xu D. MRI of atherosclerosis in clinical trials. NMR in Biomedicine: An International Journal Devoted to the Development and Application of Magnetic Resonance In vivo. 2006; 19:636-654.
8. Ronneberger O, Fischer P, Brox T. U-net: Convolutional networks for biomedical image segmentation. In: Proceedings of the International Conference on Medical image computing and computer-assisted intervention Conference 2015; Springer, 2015. p. 234-241.
9. Badrinarayanan V, Kendall A, Cipolla R. Segnet: A deep convolutional encoder-decoder architecture for image segmentation. Ieee T Pattern Anal. 2017; 39:2481-2495.
Figures
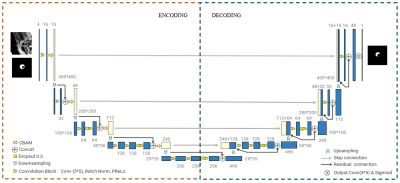
Architecture
of the proposed CNN-based Vessel-SegNet model
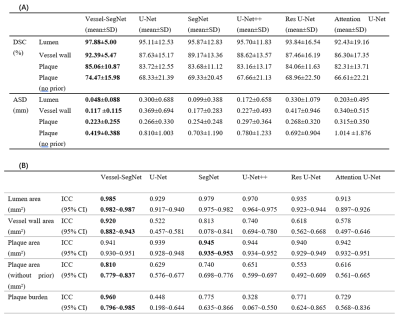
(A) Lumen, vessel wall and plaque segmentation results of each model. (B) Quantitative
assessment results of each model
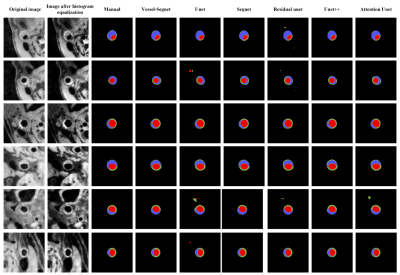
Representative lumen, vessel wall and plaque segmentation results of different models
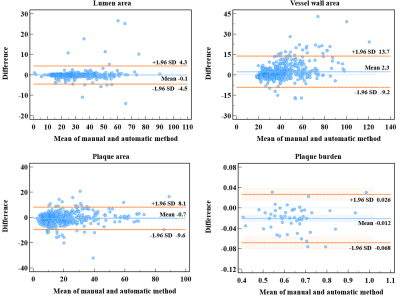
Brand-Altman
plots between the manual and automatic methods
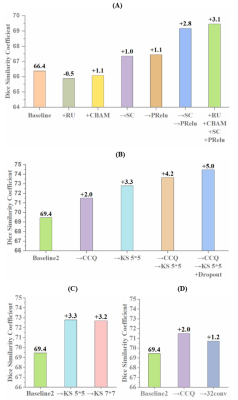
Performance of different modules and combinations; (A) Part I ablation experiment, (B) Part II ablation experiment, (C)&(D) Further explore the kernel size and the number of convolution blocks in Part II.
Note: RU: residual unit, CBAM: Convolutional Block Attention Module, SC: stride convolution, PReLU: parametric rectified linear unit, CCQ: changeable convolution quantity, KS: kernel size
DOI: https://doi.org/10.58530/2023/1509