1508
Automatic segmentations for carotid vessel lumen and wall with arterial calcifications using spatially registered black- and gray-blood images1Center Laboratory, The Third Affiliated Hospital of Nanchang University, Nanchang, China, 2Radiology Department, The First Hospital of Nanchang City, Nanchang, China
Synopsis
Keywords: Vessel Wall, Segmentation
We investigated automatic segmentations for the carotid vessel lumen and wall with the presence of arterial calcifications using spatially registered black- and gray-blood images. CS-siBLAG sequence was used to provide black- and gray-blood images. A K-means algorithm was employed to segment carotid artery lumen on black-blood images and calcifications on gray-blood images. A distance transform and an active contours model was used to segment the vessel wall. This method improves lumen segmentation, since it avoids the over-segmentation of vessel lumen by means of subtracting calcifications obtained on gray-blood images from the vessel lumen segmentation obtained on black-blood images.Introduction
The accurate segmentation of vessel wall and lumen on black-blood images is important for clinically quantitative assessment of plaques, which is essential for evaluating plaque progression and treatment effects (1). However, lumen segmentation that solely performs on black-blood images probably delivers inaccurate results when there exist superficial calcifications in carotid plaques. The reason is the hypointense appearance of superficial calcifications make them difficult to distinguish from the artery lumen (2), leading to overestimation of lumen area. In this study, the segmentation was carried out on spatially registered black- and gray-blood images to achieve more accurate segmentation for images with the presence of arterial calcifications.Materials and Methods
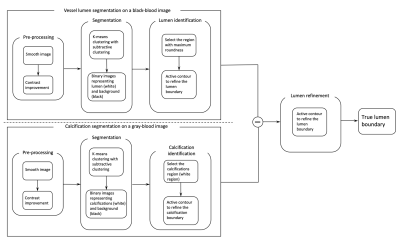
A compressed sensing (CS) based simultaneous 3D black- and gray-blood imaging sequence (CS-siBLAG) (2) was implemented to provide spatially registered black- and gray-blood images in one single and short scan. A pseudo-centric phase encoding order (3) was used to fill the undersampled k-spaces. We used an automatic lumen segmentation algorithm (4) to obtain initial lumen segmentation on black-blood images. A K-means algorithm with subtractive clustering was employed to separate regions with similar intensity. Binary images are then obtained by thresholding the K-means clustering outcome. Then one circularity criteria function (4) is used to evaluate each region (potential lumen) in the binary image. Next, we used the same K-means algorithm to segment calcifications on gray-blood images. A subtraction operation between the areas of vessel lumen and calcifications was then carried out, and an active contour method (5) was applied to the subtraction to achieve true lumen boundary. Figure 1 shows the diagram of the proposed lumen segmentation.For vessel wall segmentation, first a distance transform (6) was carried out to detect the boundary of lumen and generate the initial contour of vessel wall. Then, a Snake model with an ellipse constraint and a weighted external energy was employed to expand the initial contour towards the correct boundary of the carotid artery.
MR images were acquired using a clinical 3 T scanner (Signa TM; GE Medical Systems, Milwaukee, WI). One patient (female, 75 years) was recruited. The imaging parameters of CS-siBLAG were TR/TE of 6.2/2.9 ms, flip angle 6°, FOV 180 × 180 mm2, receiver bandwidth 244 Hz/pixel, matrix size 256 × 256, interpolated to 512 × 512, slice thickness 1.4 mm, number of slices 48, and signal averages 1. Two-fold CS acceleration factor was used. The scan time was 106 seconds. A 3D TOF sequence was also implemented as reference to verify the locations of calcifications. The 3D TOF imaging parameters were TR/TE 29/2 ms, flip angle 20°, field of view 180 × 180 mm2, receiver bandwidth 150 Hz/pixel, matrix size 256 × 256, interpolated to 512 × 512, signal averages 1, slice thickness 1.4 mm, number of slices 48. The 3D TOF sequence was fully sampled, and the scan time was 360 seconds.
Results
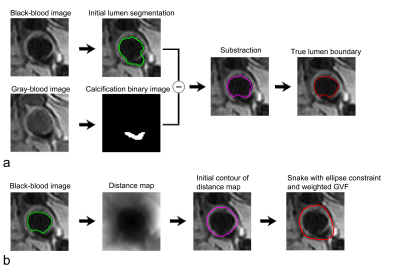
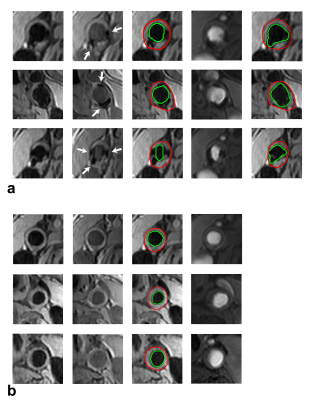
The segmentation of vessel lumen with the presence of arterial calcifications in dual-blood contrast images is depicted in Fig. 2a. The lumen is segmented on the black-blood image (green contour), while the calcification is segmented on the gray-blood image (binary image). The vessel lumen in magenta contour is obtained by subtracting the calcification from the initial vessel lumen segmentation. An active contour is then applied to achieve true lumen boundary (red contour). Figure 2b shows the approach for segmenting the carotid vessel wall. The identified lumen obtained in Fig. 2a is submitted to the vessel wall segmentation (green contour in Fig. 2b). Next, a distance map is generated to obtain the initial contour of vessel wall (magenta contour). The outer vessel wall boundary (red contour) is then detected by the expansion of the initial contour with a Snake model with an ellipse constraint and a weighted external energy.Figure 3a shows the segmentation of the carotid vessel wall and lumen. The first and second columns are the black- and gray-blood images. The calcifications signals are depicted ambiguous in black-blood images but clear in gray-blood images (arrows in the second column). The images in the third column illustrate the lumen contours (in green) and the vessel wall contours (in red) obtained by the proposed method. The lumen segmentation evidently excludes the calcifications signals. The images of 3D TOF corroborate the presence of these calcifications (the fourth column). The images in the fifth column show the segmentation only using black-blood images, and the overestimated lumen contour can be easily seen on the images. Figure 3b shows the segmentation on images without arterial calcifications. The images in the third column show the lumen contours (in green) and the vessel wall contours (in red).
Discussion
Gray-blood images can be used for the segmentation of calcifications, which is beneficial to achieve true vessel lumen boundary. This method avoids the over-segmentation of vessel lumen by means of subtracting calcifications obtained on gray-blood images from the vessel lumen segmentation obtained on black-blood images. Therefore, the method can be a promising tool for clinically morphological and quantitative assessment of plaques.Acknowledgements
This work was supported by the National Natural Science Foundation of China (81760311).References
1. Xu W, Yang X, Li Y, Jiang G, Jia S, Gong Z, Mao Y, Zhang S, Teng Y, Zhu J, He Q, Wan L, Liang D, Li Y, Hu Z, Zheng H, Liu X, Zhang N. Deep Learning-Based Automated Detection of Arterial Vessel Wall and Plaque on Magnetic Resonance Vessel Wall Images. Front Neurosci 2022;16:888814.2.
2. Li B, Li H, Kong H, Dong L, Zhang J, Fang J. Compressed sensing based simultaneous black- and gray-blood carotid vessel wall MR imaging. Magn Reson Imaging 2017;38:214-223.3.
3. Li B, Li H, Li J, Zhang Y, Wang X, Zhang J, Dong L, Fang J. Relaxation enhanced compressed sensing three-dimensional black-blood vessel wall MR imaging: Preliminary studies. Magn Reson Imaging 2015;33(7):932-938.4.
4. Jodas DS, Pereira AS, Tavares J. Automatic segmentation of the lumen region in intravascular images of the coronary artery. Med Image Anal 2017;40:60-79.5.
5. Chan TF, Vese LA. Active contours without edges. IEEE Trans Image Process 2001;10:266-277.
Figures