1500
Characterizing pathological grade and histological variant of bladder urothelial carcinoma with a continuous-time random walk diffusion model1Department of Radiology, Peking University First Hospital, Beijing, China, 2MR Collaboration, Central Research Institute, Shanghai United Imaging Healthcare, Shanghai, China
Synopsis
Keywords: Urogenital, Diffusion/other diffusion imaging techniques
Accurately determining the histologic grade and variant status is essential. In this study, the diagnostic value of the CTRW model in characterizing histological grade or histological variants was evaluated and compared with ADC. The optimal diagnostic performance of CTRW diffusion model suggested it could provide more diagnostic value than conventional ADC not only for pathological grading but also for histological variant of bladder urothelial carcinoma.Introduction
Bladder urothelial carcinoma with high histological grade or histological variants are usually associated with poor outcomes1-3. Accurately determining the histologic grade and variant status is essential. Transurethral resection of bladder tumour (TURBT) has been used to determine histologic grade4 and detecting histologic variant5. However, due to the sampling errors and low sensitivity, TURBT may result in inaccurate grading and lower detection rate of histological variant5,6. Diffusion-weighted imaging (DWI) can non-invasively investigate the diffusivity of water molecules within tissues7. Considering the varying structural complexity in cancer, a non-gaussian diffusion model, continuous-time random-walk (CTRW) model8 may provide a more comprehensive characterization for complex micro-environment in bladder cancer. As such, the purpose of this study is to evaluate the ability of the multi-b-value DWI with the CTRW diffusion model in determining the pathological grading and histological variant of bladder urothelial carcinoma.Methods
Totally 55 patients (45 high grades, 22 with histological variants) with pathologically confirmed bladder urothelial carcinoma were prospectively enrolled. All patients underwent the bladder MRI on a 3.0 T MRI scanner (uMR790, United Imaging Healthcare, Shanghai, China). Multi-b-value DWI with 11 b-values (b = 0, 50, 100, 200, 400, 800, 1000, 1500, 2000, 2500, 3000 s/mm2) was acquired using a single-shot spin-echo echo planar imaging (EPI) sequence. Three CTRW model parameters, including an anomalous diffusion coefficient Dm, and two parameters related to temporal and spatial diffusion heterogeneity α and β9, respectively, were obtained by a voxel-by-voxel non-linear fitting based on the Levenberg–Marquardt algorithm. The apparent diffusion coefficient (ADC) was calculated by DWI with b-value=0 and 800. Regions of interest (ROIs) analysis was performed to cover the solid tumor and exclude the cystic or necrotic areas. Then the mean value of CTRW parameters (Dm, α, and β) and ADC were computed over the tumor ROIs. Mann-Whitney U test was used to compare the difference of Dm, α, and β between (1) high-grade (HG) and low-grade (LG) urothelial carcinoma; (2) pure urothelial cancer (UC) and urothelial cancer with histological variant (HV). Binary logistic analysis was performed to combine all the CTRW parameters. Receiver operating characteristic (ROC) analysis was employed to evaluate the diagnostic performance of single CTRW parameter, ADC and their combination (Dm, α, and β) in characterizing pathological grading and histological variant of BCa. P-value< 0.05 was considered as statistically significant.Results
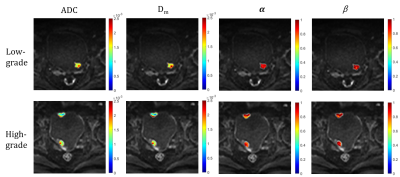
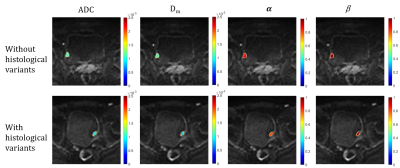
Representative images of LG, HG, UC and HV are shown in Figure 1 and Figure 2. HG showed significantly lower Dm, and α values than LG (Dm: 1.09±0.26 vs. 1.92±0.70 μm2/ms; α: 0.73±0.13 vs. 0.89±0.07; p<0.001). HV showed significantly lower Dm and α values than UC (Dm: 0.99±0.27 vs. 1.40±0.54 μm2/ms; α: 0.69±0.14 vs. 0.81±0.10; p<0.001). β was not statistically different (p=0.62, 0.36). The area under the curve (AUC) of Dm, α, and ADC for HG was 0.94, 0.90, and 0.95, respectively. The AUC of Dm, α, and ADC for HV was 0.80, 0.71, and 0.78, respectively. Combined Dm, α, and β had slightly better performance than ADC for HG and HV diagnosis, with the AUC values of 0.96 and 0.80, respectively.Discussion
Similar to conventional ADC, Dm reflects tissue cellularity, cell membrane integrity and extracellular space. Lower Dm values in HG and HV may be related to the increased cellularity and decreased extracellular space tortuosity10. The parameters α and β was reversely related to the degree of temporal and spatial diffusion heterogeneity, respectively8,9. In our study, higher α values in LG and UC reflected the more complex and heterogeneous structure in temporal dimension, which meant the time for water molecules to take a move was longer. The diagnostic value of the CTRW model in characterizing histological grade or histological variants was evaluated and compared with ADC. Incorporating all CTRW parameters could slightly improve the diagnostic performance, suggesting the clinical potential of CTRW diffusion model in determining histological grade or histological variants of bladder urothelial carcinoma.Conclusion
The CTRW diffusion model could provide more diagnostic value than conventional ADC not only for pathological grading but also for histological variant of bladder urothelial carcinoma.Acknowledgements
No acknowledgementsReferences
1. Takemoto, Kenshiro et al. “The Impact of Histological Variant on Oncological Outcomes in Patients With Urothelial Carcinoma of the Bladder Treated With Radical Cystectomy.” Anticancer research vol. 40,8 (2020): 4787-4793. doi:10.21873/anticanres.14481
2. Xylinas, Evanguelos et al. “Impact of histological variants on oncological outcomes of patients with urothelial carcinoma of the bladder treated with radical cystectomy.” European journal of cancer (Oxford, England : 1990) vol. 49,8 (2013): 1889-97. doi:10.1016/j.ejca.2013.02.001
3. Reisz, Peter A et al. “Management of High-grade T1 Urothelial Carcinoma.” Current urology reports vol. 19,12 103. 26 Oct. 2018, doi:10.1007/s11934-018-0850-8
4. Kim, Lawrence H C, and Manish I Patel. “Transurethral resection of bladder tumour (TURBT).” Translational andrology and urology vol. 9,6 (2020): 3056-3072. doi:10.21037/tau.2019.09.38
5. La Croce, Giovanni et al. “The Accuracy of Transurethral Bladder Resection in Detecting Bladder Cancer Histological Variants and Their Prognostic Value at Radical Cystectomy.” Journal of clinical medicine vol. 11,3 550. 22 Jan. 2022, doi:10.3390/jcm11030550
6. Ark, Jacob T et al. “Incidence and predictors of understaging in patients with clinical T1 urothelial carcinoma undergoing radical cystectomy.” BJU international vol. 113,6 (2014): 894-9. doi:10.1111/bju.12245
7. Tang, Lei, and Xiaohong Joe Zhou. “Diffusion MRI of cancer: From low to high b-values.” Journal of magnetic resonance imaging : JMRI vol. 49,1 (2019): 23-40. doi:10.1002/jmri.26293
8. Ingo, Carson et al. “On random walks and entropy in diffusion-weighted magnetic resonance imaging studies of neural tissue.” Magnetic resonance in medicine vol. 71,2 (2014): 617-27. doi:10.1002/mrm.2470 9. Karaman, M Muge et al. “Quartile histogram assessment of glioma malignancy using high b-value diffusion MRI with a continuous-time random-walk model.” NMR in biomedicine vol. 34,4 (2021): e4485. doi:10.1002/nbm.4485
10. Wang, Yanchun et al. “Comparison of the Diagnostic Value of Monoexponential, Biexponential, and Stretched Exponential Diffusion-weighted MRI in Differentiating Tumor Stage and Histological Grade of Bladder Cancer.” Academic radiology vol. 26,2 (2019): 239-246. doi:10.1016/j.acra.2018.04.016
Figures