1499
Evaluation of Amide Proton Transfer Imaging for Bladder Cancer Histopathologic Features: A Comparative Study with Diffusion- Weighted Imaging1Tongji Hospital, School of Medicine, Tongji University, Shanghai, China, 2Philips Healthcare, Shanghai, China
Synopsis
Keywords: Urogenital, Bladder
Amide proton transfer (APT) imaging is an emerging chemical exchange saturation transfer (CEST)-based MRI technique that is sensitive to mobile proteins and peptides in tissue and has drawn considerable attention in the field of cellular and molecular imaging.In the present work, our result demonstrates that APT imaging can predict tumor grade and muscular invasion in bladder cancer, the diagnostic performance for evaluating muscular invasion is better than that of diffusion-weighted imaging (DWI) and adding APT imaging to DWI significantly improved the diagnostic accuracy for evaluating muscular invasion versus DWI alone, thus opening new research avenues in this field.Introduction
In the past years, studies have demonstrated essential roles of magnetic resonance imaging in bladder cancer (BCa) diagnosis, grade and stage. However, these morphological and functional multiparametric MRI sequences cannot reflect the pathophysiology of the tissue at the molecular level, and given the limitations of current clinical approaches, new molecular MRI techniques are needed for bladder cancer. Amide proton transfer (APT) imaging, based on chemical exchange saturation transfer (CEST), is an emerging molecular MRI method to detect mobile proteins and peptides in tissue. Many studies have investigated APT imaging and found that it has potential clinical application value for differentiating between benign and malignant lesions, grading tumors, and evaluating the efficacy of chemotherapy [1-12]. However, the role of APT imaging to grade and assess muscular invasion of bladder cancer is still a mistery; therefore, we aimed to explore whether APT imaging could differentiate low-grade from high-grade tumors and evaluate the muscular invasion of BCa and to compare its ability to assess the grade and muscular invasion of BCa with that of DWI.Method
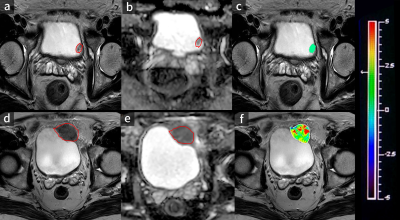
Forty-eight patients diagnosed with BCa confirmed by histopathological findings who underwent magnetic resonance (MR) imaging, including APT imaging and DWI (b=0, 1000 s/mm2), were enrolled in this study. MTRasym (asymmetric magnetization transfer ratio) was defined as the magnetization transfer asymmetry at 3.5 ppm. MTRasym and apparent diffusion coefficients (ADCs) were compared between the low- and high-grade groups and between non-muscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC) in terms of the areas under the receiver operating characteristic curves (AUCs).Results
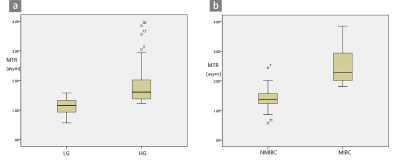
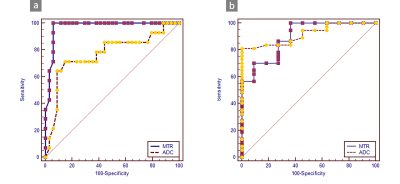
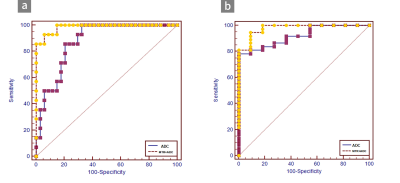
MTRasym values were significantly higher in patients with high-grade bladder tumors than in those with low-grade tumors (P=0.000) and in MIBC than in NMIBC (P=0.000). The AUCs of MTRasym were significantly larger than those of ADC for differentiating MIBC from NMIBC (P=0.016), while the comparison of the AUCs for differentiating low-grade from high-grade tumors was ADC >MTRasym (P=0.75). Adding APT imaging to DWI significantly improved the diagnostic accuracy for differentiating MIBC from NMIBC versus DWI alone (P=0.013).Discussion
In this study, we found that MTRasym values were significantly higher in patients with high-grade bladder tumors than in those with low-grade bladder tumors and in those with MIBC than in those with NMIBC. MIBC is heterogeneous with atypical cells, which are characterized by endothelial proliferation, vascular hyperplasia, hemorrhage and necrosis. However, NMIBC consists of more homogeneous clusters of well-differentiated cells [13]. Malignant tumors present obvious cell and structural atypia, including an increase in the nucleocytoplasmic ratio, megakaryocytes, ribosomes and malformed nuclei [7]. These results support the hypothesis that the active metabolism of tumors produces large amounts of proteins and validate that APT imaging can be a tumor proliferative index. Our study indicated that APT imaging could perform better than DWI in evaluating muscular BCa. APT imaging is potentially sensitive to the microstructural molecular changes that occur prior to macroscopic changes in gross morphology without contrast. Microcirculation perfusion, motion distortion, susceptibility and different b values might affect ADC values. Moreover, the addition of APT imaging to DWI significantly improved the diagnostic accuracy for differentiating MIBC from NMIBC versus DWI alone. This result indicates that DWI combined with APT imaging could be an efficient method to predict muscular invasion compared to DWI alone. APT imaging could provide additional information for the tumor microenvironment compared to DWI. The microvasculature of BCa cells might increase due to the increasing density of the tumor, which leads to an increase in additional immature capillary networks and greater blood perfusion and may thus increase the APT signal intensity. Similarly, the active metabolism of tumors leads to denser density, which limits the diffusion of water molecular. Therefore, the combination of functional MRI and molecular MRI can be an effective and noninvasive method to predict the muscular invasion of BCa.Conclusion
In conclusion, APT imaging alone can be helpful for differentiating low-grade from high-grade tumors and evaluating the muscular invasion of BCa, and adding APT imaging to conventional DWI could significantly improve the diagnostic performance for evaluating the muscular invasion of BCa.Acknowledgements
This work was supported by the National Natural Science Foundation of China [grant numbers 81901733, 81974274]; Excellent Discipline Reserve Talent Program of Tongji Hospital Affiliated to Tongji University [grant number HBRC2109]; Key discipline construction project of the three-year action plan of Shanghai public health system [grant number GWV-10.1-XK9]; Shanghai "Rising Stars of Medical Talent" Youth Development Program-Youth Medical Talents-Medical Imaging Practitioner Program [grant number SHWRS(2020)_087]; The Fundamental Research Funds for the Central Universities (grant number 22120210568) .References
1. Zhou J, Tryggestad E, Wen Z, et al. Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and Peptides. Nature Med 2011;17:130-134.
2. Zhao X, Wen Z, Huang F, et al . Saturation power dependence of amide proton transfer image contrasts in human brain tumors and strokes at 3T. Magn Reson Med 2011;66:1033-1041.
3. Yuan J, Chen S, King AD, et al. Amide proton transfer-weighted imaging of the head and neck at 3 T:a feasibility study on healthy human subjects and patients with head and neck cancer. NMR Biomed 2014;27: 1239-1247.
4. Zhou J, Zhu H, Lim M, et al. Three-dimensional amide proton transfer MR imaging of gliomas: initial experience and comparison with gadolinium enhancement . J Magn Reson Imaging 2013;38: 1119-1128.
5. Jia G, Abaza R, Williams JD, et al. Amide proton transfer MR imaging of prostate cancer: a preliminary study. J Magn Reson Imaging 2011;33:647-654.
6. Takayama Y, Nishie A, Togao O, et al. Amide Proton Transfer MR Imaging of Endometrioid Endometrial Adenocarcinoma: Association with Histologic Grade. Radiology 2018;286:909-917.
7. Li B, Sun H, Zhang S, Wang X, Guo Q. Amide proton transfer imaging to evaluate the grading of squamous cell carcinoma of the cervix: A comparative study using 18 F FDG PET. J Magn Reson Imaging 2019; 50:261-268.
8. Li B, Sun H, Zhang S, Wang X, Guo Q .The utility of APT and IVIM in the diagnosis and differentiation of squamous cell carcinoma of the cervix: A pilot study. Magn Reson Imaging 2019;63:105-113.
9. Nishie A, Takayama Y, Asayama Y, et al. Amide proton transfer imaging can predict tumor grade in rectal cancer. Magn Reson Imaging 2018;51:96-103.
10. Nishie A, Asayama Y, Ishigami K, et al. Amide proton transfer imaging to predict tumor response to neoadjuvant chemotherapy in locally advanced rectal cancer. J Gastroenterol Hepatol 2019;34:140-146.
11. Dula, AN, Arlinghaus, LR, Dortch RD, et al. Amide proton transfer imaging of the breast at 3 T: establishing reproducibility and possible feasibility assessing chemotherapy response. Magn Reson Med 2013;70:216-224.
12. Ohno Y, Yui M, Koyama H, et al. Chemical Exchange Saturation: Preliminary Results for Differentiation of Malignant and Benign Thoracic Lesions. Radiology 2016;279:578-589.
13.O'Brien T, Cranston D, Fuggle S, Bicknell R, Harris AL. Different angiogenic pathways characterize superficial and invasive bladder cancer. Cancer Res 1995;55:510-513.
Figures