1494
Preoperative assessment of vessels encapsulating tumor clusters (VETC) in hepatocellular carcinoma using IVIM diffusion-weighted imaging
Chenhui Li1, Jinhuan Xie1, Liling Long1, Huiting Zhang2, and Yang Song2
1The First Affiliated Hospital of Guangxi Medical University, Nanning, China, 2MR Scientific Marketing, Siemens Healthineers, Shanghai, China
1The First Affiliated Hospital of Guangxi Medical University, Nanning, China, 2MR Scientific Marketing, Siemens Healthineers, Shanghai, China
Synopsis
Keywords: Cancer, Liver
Vessels encapsulating tumor clusters (VETC) is a powerful predictor of a poor prognosis in patients with hepatocellular carcinoma (HCC) .Therefore, preoperative radiologic prediction of these histopathological results can allow optimized management of HCC and help improve long-term survival for patients.However,to our knowledge, no published studies have investigated preoperative VETC patten of HCC using IVIM parameters and comparing with DWI. Our study indicated that IVIM-derived D* provides a non-invasive analytical approach for preoperative predicting VETC of HCC.ADC value were not predictive for VETC.Introduction
Vessels encapsulating tumor clusters (VETC) is a powerful predictor of a poor prognosis in patients with hepatocellular carcinoma (HCC) 1,2, because it provides the route for tumor cells to access the portal or systemic circulation. Unfortunately, information regarding VETC status and tumor differentiation is not routinely available preoperatively, thus, limiting their clinical utility in decision making. Therefore, preoperative radiologic prediction of these histopathological results can allow optimized management of HCC and help improve long-term survival for patients. Conventional DWI supposes the Gaussian water diffusion, and thus cannot distinguish between the diffusion of water molecules and the perfusion of blood. Specifically, intravoxel incoherent motion (IVIM) DWI can simultaneously quantify the diffusion of water molecules and microcirculation perfusion in living tissues. Therefore, it can compensate the limitations of conventional DWI3. It has been reported that IVIM can Improve tumor characterization and grading compared with traditional DWI4. However, to our knowledge, no published studies have investigated preoperative VETC patten of HCC using IVIM parameters and comparing with DWI. The aim of this study was to prospectively evaluate the diagnostic efficacy of IVIM in predicting VETC of HCC with comparison to the conventional DWI.Methods
The prospective study was approved by our Medical Ethics Committee. 104 patients with HCC confirmed by histopathological results were recruited between March 2021 to April 2022. All patients underwent preoperative routine MR and IVIM sequence examination on a 3T MRI scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) with a 16 channel body coil. Besides conventional T1WI and T2WI sequences, a research application multi-b DWI using single-shot echo-planner imaging with integrated-shimming sequence was performed with breath navigation under free breathing. The parameters were as follows: 8 b-values: 0 , 20 , 50 , 100 , 150 , 200 , 600 and 1000 s/mm2; TR: 4900 ms, TE: 57ms, FOV: 380 mm × 261 mm, matrix: 88×128, layer thickness: 5.0mm, bandwidth: 2442 Hz/pixel, acceleration factor: 2. The apparent diffusion coefficient (ADC) map and IVIM-derived parameters maps of molecular diffusion coefficient (D), perfusion fraction (f), and perfusion-related diffusion coefficient (D*) were calculated from all 8 b-values. All the quantitative images were analyzed by two independent radiologists who were blinded to the histopathological results. The radiologist drew freehand ROI to outline the tumor around the tumor margin on the original DW images (b = 1000 s/mm2) on whole tumor volume,which was the largest lesion, and tried to avoid the obvious hemorrhage, calcified and necrotic areas. The ROIs then were copied to all other quantitative maps. The mean value of each parameter for the whole tumor was used for further statistical analysis. The surgically resected hepatic specimens were used for the pathological evaluation. Regarding the CD34 evaluation, unequivocal immunoreactivity of a continuous lining around tumor clusters was defined as VETC. The area of VETC was semi-quantitatively evaluated, and 55% was applied as the optimal cutoff value to further divide HCC patients into the VETC+ (Fig. 1) or VETC− (Fig. 2) group as previously reported 2. Continuous variables were firstly checked for homogeneity by using F test, and independent sample t test or t′ test was used for the continuous variables. Interobserver agreement toward the diffusion parameters were checked by using the intra-class correlation coefficient (ICC) with the two-way random method. Receiver operating characteristics (ROC) curve analyses were performed to evaluate the diagnostic efficiency for predicting VETC. All statistical analyses were performed by using a statistical software package SPSS 23.0 (SPSS Inc.).Results
Results were reported in Table 1. Of all the 104 HCCs, VETC was present (VETC+) with 46 (44.2%) HCCs and absent (VETC-) with 58 (55.8%) HCCs. Only the D* value was significantly higher in the VETC+ group than that in the VETC− group (all p < 0.001, Fig.1 and Fig.2). No statistical significance was observed for ADC, D, and f (p>0.05) between the two groups. The D* value for VETC of HCC showed an area under ROC curves of 0.863 (95% CI, 0.782–0. 0.923) (Fig. 3).Discussion
Our study indicated that D* from IVIM parameters superior to ADC in detecting VETC of HCC. According to IVIM theory, each IVIM parameter represents a specific biological property in a tissue; D stands for tissue cellularity, f for the fractional volume of microcapillary perfusion, and D* for flow velocity that is defined by flow velocity and the mean capillary segment length 5. VETC+ and VETC- correspond to morphologic features of micro-vessels in two very different tumors, respectively. Immunohistochemical and immunofluorescent staining show that vascular endothelial cells forms a wider lumen6, which indicates faster blood flow or longer segment length and may lead to a higher D* of VETC+ tumors, as shown in our study. However, no significant differences in the f value were found between VETC+ and VETC-. This might be because the D* value and the f value represent different aspects of perfusion, with D* representing the flow rate of blood in the microvessels and the f value representing the blood-carrying capacity of the capillaries 7. And D can not reflect the changes in the vascular microstructure compared to the D*.Conclusion
IVIM-derived D* provides a non-invasive analytical approach for preoperative predicting VETC of HCC. ADC value were not predictive for VETC.Acknowledgements
No acknowledgement found.References
- Ding T, Xu J, Zhang Y, et al. Endothelium-coated tumor clusters are associated with poor prognosis and micrometastasis of hepatocellular carcinoma after resection. Cancer. 2011 Nov; 117(21):4878-4889. DOI: 10.1002/cncr.26137. PMID: 21480209.
- Renne SL, Woo HY, Allegra S, Rudini N, Yano H, Donadon M, Viganò L, Akiba J, Lee HS, Rhee H, Park YN, Roncalli M, Di Tommaso L. Vessels Encapsulating Tumor Clusters (VETC) Is a Powerful Predictor of Aggressive Hepatocellular Carcinoma. Hepatology. 2020 Jan; 71(1):183-195. doi: 10.1002/hep.30814. Epub 2019 Aug 9. PMID: 31206715.
- Yoon JH, Lee JM, Yu MH, Kiefer B, Han JK, Choi BI. Evaluation of hepatic focal lesions using diffusion-weighted MR imaging: comparison of apparent diffusion coefficient and intravoxel incoherent motion-derived parameters. J Magn Reson Imaging. 2014 Feb; 39(2):276-85. doi: 10.1002/jmri.24158. Epub 2013 Apr 30. PMID: 23633178.
- Peng J, Zheng J, Yang C, Wang R, Zhou Y, Tao YY, Gong XQ, Wang WC, Zhang XM, Yang L. Intravoxel incoherent motion diffusion-weighted imaging to differentiate hepatocellular carcinoma from intrahepatic cholangiocarcinoma. Sci Rep. 2020 May 7; 10(1):7717. doi: 10.1038/s41598-020-64804-9. PMID: 32382050; PMCID: PMC7206040.
- Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988 Aug; 168(2):497-505. doi: 10.1148/radiology.168.2.3393671. PMID: 3393671.
- Fang JH, Zhou HC, Zhang C, Shang LR, Zhang L, Xu J, Zheng L, Yuan Y, Guo RP, Jia WH, Yun JP, Chen MS, Zhang Y, Zhuang SM. A novel vascular pattern promotes metastasis of hepatocellular carcinoma in an epithelial-mesenchymal transition-independent manner. Hepatology. 2015 Aug; 62(2):452-65. doi: 10.1002/hep.27760. Epub 2015 Apr 22. PMID: 25711742.1.
- Le Bihan D, Turner R. The capillary network: a link between IVIM and classical perfusion. Magn Reson Med. 1992 Sep; 27(1):171-8. doi: 10.1002/mrm.1910270116. PMID: 1435202.
Figures
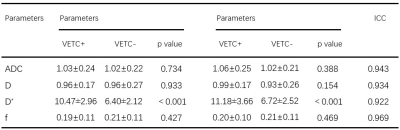
Table1.
ADC
and IVIM parameters between the VETC+ and VETC- groups and the agreements
between two radiologists
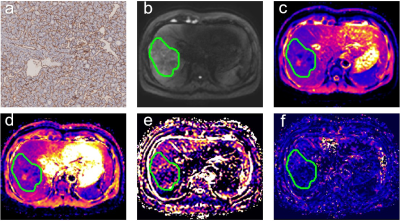
Fig 1. Surgically confirmed HCC with VETC positive in a 50-year-old man.(a) Immunohistochemical staining of CD34 in HCC tissues. (b) Original DW images (b = 1000 s/mm2) . (c) ADC map, ADC value for the lesion was 1.02 × 10−3 mm2/s. (d) D map, D value for the lesion was 1.00 × 10−3 mm2/s. (e) f map, f value was 0.14 × 100%. (f) D* map, D* value for the lesion was 13.02 × 10−3 mm2/s.
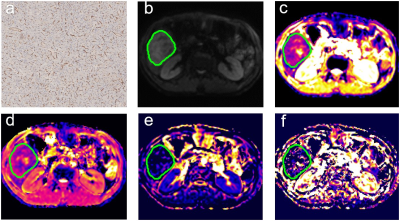
Fig
2. Surgically
confirmed HCC with VETC negative in a 44-year-old man.(a) Immunohistochemical
staining of CD34 in HCC tissues. (b) Original DW images (b = 1000 s/mm2) . (c)
ADC map, ADC value for the lesion was 1.12 × 10−3 mm2/s.
(d) D map, D value for the lesion was 1.20 × 10−3 mm2/s.
(e) f map, f value was 0.03 × 100%. (f) D*map, D* value for the lesion was 5.37 × 10−3 mm2/s.
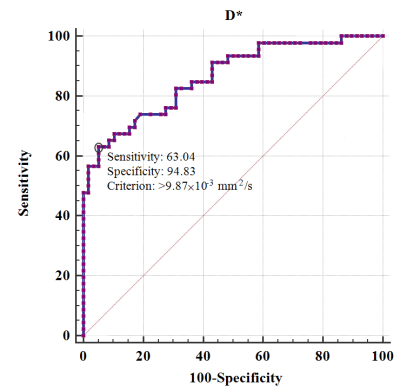
Fig 3. ROC
curves of D* to distinguish VETC+ and VETC- HCCs. AUC value was 0.863 (95% CI, 0.782–0.923)
with the optimal cutoff value of 9.87 × 10−3 mm2/s
DOI: https://doi.org/10.58530/2023/1494