1491
Early monitoring of radiation treatment based on tumor redox status using in vivo dynamic nuclear polarization MRI
Fuminori Hyodo1, Norikazu Koyasu1, Ryota Iwasaki1, Hinako Eto2, Abdelazim Elsayed Elhelaly1, Hiroyuki Tomita1, Takashi Elsayed Mori1, Masaharu Murata2, Yoshifumi Noda1, Hiroki Kato1, and Masayuki Matsuo1
1Gifu University, Gifu, Japan, 2Kyushu University, Fukuoka, Japan
1Gifu University, Gifu, Japan, 2Kyushu University, Fukuoka, Japan
Synopsis
Keywords: Cancer, Hyperpolarized MR (Non-Gas), redox, DNP MRI, radiation
In vivo dynamic nuclear polarization-MRI (DNP-MRI, also called OMRI, PEDRI) using carbamoyl-PROXYL(CmP) as a redox sensitive DNP probe enables the accurate monitoring of the tissue redox status. We found that the redox status decreases 1 day after radiation treatment, and the decay of redox status occurs before any micro- or macroscopic changes in tumor morphology and pyruvate metabolism based on the Warburg effect. This decay of redox status can also be associated with the decreased production of intratumor reducing redox molecules such as GSH and AsA.Introduction
Radiotherapy plays a very important role as one of the major treatments for cancer. In general, radiation treatment effectiveness is assessed through the observation of morphological changes with CT or MRI images after treatment. However, the process of evaluating treatment outcomes in clinical settings can be very time consuming, and even unsafe when treatments are found to be not fully effective. Therefore, it is necessary to develop a technological tool that can determine the response to treatment in the early stages of the disease. We have been investigating the redox imaging method using a in vivo DNP-MRI system. In in vivo DNP-MRI, also known as Overhauser enhanced MRI (OMRI) or proton-electron double resonance imaging (PEDRI) EPR irradiation at the resonant frequency of the in vivo free radical molecule induces DNP, and, subsequently, increases the MRI signal. Overproduction of ROS can also be triggered by irradiation, which may alter the redox status in tumor tissue. In order to early detect the effectiveness of radiotherapy, it is necessary to detect the degree to which the redox status is altered by radiation treatment before the acquisition of morphological changes using 1.5 T animal MRI. DNP-MRI can sensitively detect changes in the redox status of tissues1). Therefore, the purpose of this study is to evaluate the early alteration of redox status induced by radiation treatment in tumor tissues using the in vivo DNP-MRI/CmP probe method(Fig.1A). In addition, the metabolic responses of redox status and the Warburg effect, which pyruvate dominantly metabolizes to lactate in cancer cells instead of the normal energy metabolism, are compared using the two DNP methods of in vivo DNP-MRI and hyperpolarized 13C pyruvate MRS2)Methods
MIA PaCa-2 human pancreatic carcinoma cells were purchased from ATCC. In vivo free radical imaging was performed with a low-field DNP-MRI system (Keller). The external magnetic field B0 for EPR irradiation and MRI was fixed at 15 mT, and the radiofrequencies of EPR irradiation and MRI were 458 MHz and 689 kHz, respectively. A single-turn surface coil (inner diameter 19 mm) for EPR irradiation was used for tumor imaging in this study. The in vivo DNP-MRI scanning of the tumor-bearing legs was started immediately after the intravenous injection of carbamoyl-PROXYL (300 mM). Pharmacokinetic DNP-MRI images were obtained at 0.5, 2, 4, 6, 8, 10, and 14 min after administration. The decay rate was calculated according to the intensity changes in tumor images during the time course from 0.5 to 14 minutes after injection of CmP. For the dissolution DNP study, a final concentration of 80 mM Hyperpolarized 13C-pyruvate was injected intravenously into mice at a dose of 15μL /g body weight within 3–5 sec. Spectrum acquisition was accomplished using the 1.5 T MR scanner, equipped with a dual tuned 1H/13C volume transmit-receive radiofrequency coil. Tumors were irradiated with 5 Gy in an X-ray medical linear accelerator (LINAC), Primus (Siemens Healthcare, Malvern, PA, USA).The GSH and AsA assays were performed using high-performance liquid chromatography with electrochemical detection. The effect of GSH on CmP redox reaction following the generation of hydroxyl radicals was monitored by EPR spectroscopy.Results
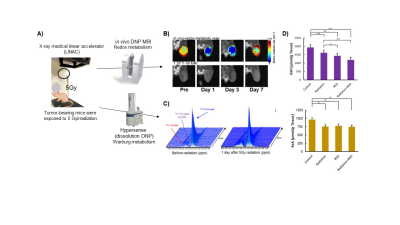
To monitor the response of tumor redox status after irradiation, tumor-bearing mice were exposed to 5 Gy irradiation and in vivo DNP-MRI was performed at 1, 3, 7 days after intravenous injection of CmP as a DNP redox probe. The distribution of the enhanced image area was observed in the tumor starting from 0.5 min post-injection. In vivo redox maps that reveal the redox status in each pixel were calculated from pharmacokinetic DNP images, and the spatiotemporal alterations of redox status were visualized(Fig.1B). Redox maps showed a clear reduction in the redox status even on the first day post-irradiation compared to pre-irradiation, and the redox status continued to decrease until day 3. Then, redox status on day 7 after irradiation recovered to the same level as that was before irradiation The tumor GSH level on day 1 post-irradiation was decreased compared to the control group(Fig.1D). When BSO, an inhibitor of gamma-glutamylcysteine synthetase, was administered with radiation, GSH level was further decreased. Similarly, tumor AsA concentration was significantly decreased in all treatment groups compared to the control group. The MR signal amplitude was amplified in accordance with the increase in the spectrum’s peak. Production of 13C-lactate in relation to 13C-pyruvate in tumor tissue did not change before and after irradiation at a statistically significant level(Fig.1C).Disucussion
We found that the redox status decreases after radiation treatment, and the decay of redox status occurs before any micro- or macroscopic changes in tumor morphology and lactate metabolism based on the Warburg effect. This decay of redox status can also be associated with the decreased production of intratumor reducing redox molecules such as GSH and AsA. Our findings suggest that DNP-MRI-based redox imaging would serve non-invasive assessment of redox status to monitor the tumor microenvironment and its associated changes in response to radiation treatment. Early diagnostic imaging based on redox status may provide physicians with new treatment strategies for cancer treatment.Acknowledgements
This research was supported by AMED under Grant Number JP19cm0106435h0002. This work was also supported by JSPS KAKENHI (Grant Numbers 18H02765 and 19H03358 and 20KK0253) and MEXT Quantum Leap Flagship Program (MEXT Q-LEAP) Grant Number JPMXS0120330644.References
1.Hyodo F, Eto H, Naganuma T, Koyasu N, Elhelaly AE, Noda Y, Kato H, Murata M, Akahoshi T, Hashizume M, Utsumi H, Matsuo M. In Vivo Dynamic Nuclear Polarization Magnetic Resonance Imaging for the Evaluation of Redox-Related Diseases and Theranostics Antioxid Redox Signal. 2022 Jan;36(1-3):172-184.
2. Koyasu N, Hyodo F, Iwasaki R, Eto H, Elhelaly AE, Tomita H, Shoda S, Takasu M, Mori T, Murata M, Hara A, Noda Y, Kato H, Matsuo M. Spatiotemporal imaging of redox status using in vivo dynamic nuclear polarization magnetic resonance imaging system for early monitoring of response to radiation treatment of tumor. Free Radic Biol Med. 2022 Feb 1;179:170-180.
Figures
DOI: https://doi.org/10.58530/2023/1491