1489
Using D2O-Induced 2H labeling, Deuterium MRI at 1.5T Enables In Vivo Visualization of Body Tissues and Early Assessment of Anticancer Therapies1Department of Radiology, Frontier Science for Imaging, Gifu University, Gifu, Japan, 2Department of Food Hygiene and Control, , Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt, 3Department of Radiology, School of Medicine, Gifu University, Gifu, Japan, 4Department of Tumor Pathology, Gifu University Graduate School of Medicine, Gifu University, Gifu, Japan
Synopsis
Keywords: Cancer, Deuterium, deuterium MR imaging
DMI emerged as a promising alternative in cancer metabolic imaging. We D2O-induced 2H-labeling followed by DMI to study deuterium kinetics and track tumor response to treatment at 1.5T. We succeeded to use dMRI to monitor 2H kinetics in tissues of a pancreatic carcinoma model. Higher 2H build-up was observed in tumor. Treated mice showed a significant decrease in 2H in tumor during 7 days of treatment and before anatomical changes were detectable. Tumor homogenates of treated mice also showed a significant reduction in 13C lactate production. DMI of tumors is a potential imaging method for assessment of early treatment responsesIntroduction
For the management of patients and creation of novel therapeutic regimens, accurate and non-invasive assessment of tumor response following radiation and/or chemotherapy is essential. Traditionally, anatomical imaging techniques like CT and MRI have been used to plan radiation therapy and assess tumor response. However, such imaging techniques are insufficient for the early detection of therapeutic response before a significant morphological change can be observed. Metabolic imaging of molecular processes in the living body such as PET and DNP-MRS of 13C metabolites were developed later and used in a wide range of conditions. In recent years, deuterium metabolic spectroscopy (DMS) and metabolic imaging (DMI) have been demonstrated as alternatives to metabolic techniques using 13C metabolic probes. Cancer metabolic imaging by high-field (4T-11T) MRI using deuterium-labeled molecules as a contrast agent has been reported. A promising way of this metabolic imaging approach is the follow up of the fate of orally administered deuterated glucose, as it is taken up and metabolized into different products in the different tissues such as heart, brain, pancreatic, liver, and cancer (1-5).Purpose
This study was conducted to investigate the feasibility of in vivo visualization of deuterium-labelled tissues induced by D2O and to try to understand its kinetics in the body tissue using the magnetic field strength that is widely used in clinical practice. We aimed also to verify the feasibility of visualization of tumor and early delineation of therapeutic effects based on deuterium MRI using D2O at 1.5T MR field. We studied the effects of three anticancer treatment strategies using deuterium magnetic imaging in a murine cancer xenografts of human pancreatic cancer model during the first 7 days after treatment.Methods
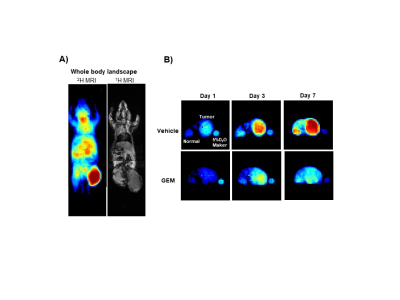
Four groups of murine models bearing xenografts of human pancreatic carcinoma, MIA PaCa-2, were prepared and allowed a free access to 30% (v/v) D2O in drinking water. Deuterium MRI scanning was conducted using a small-animals 1.5T MR scanner and deuterium coils at a frequency of 9.8MHz. Compared to a control group, mice received irradiation (single dose of 20Gy in an X-ray medical linear accelerator (LINAC) ), Bevacizumab (10 mg/kg every other day) or Gemcitabine (120 mg/kg every other day) were tested for deuterium build-up levels in tumor xenografts before and at 1-, 3- and 7-days post-treatment while allowing them free intake of the deuterated water. To confirm results of our dMRI approach of imaging, hyperpolarization of 13C pyruvate was done by a HyperSense DNP polarizer and ex vivo 13C NMR acquisition using tumor homogenates was done by a 1.4T Spinsolve Carbon benchtop NMR apparatus. T2-weighted anatomical images were also acquired using 1.5T MRI. Additional molecular and histopathological experiments were conducted to confirm the anticancer effects and find out the mechanisms behind our data.Results and Discussion
Using Deuterium MR imaging, we observed that tumor xenografts undergo a day-by-day increase in deuterium accumulation and higher levels of D2O accumulation in the tumor tissues over normal tissues were observed. This may be attributed to the fact that tumor is a highly proliferative tissue, and cell proliferation induces deuterium labeling of the newly formed cell molecules by replacing the carbon bonds with hydrogen atoms leading to the formation of new carbon-deuterium bonds that are not exchangeable with hydrogen. Upon deuterium labeling followed by dMRI, a significant reduction in the deuterium levels in tumor xenografts of mice received any of the 3 anticancer interferences were observed during the first 7 days post-treatment compared to control mice. This indicates the ability of anticancer therapy to induce cell apoptosis, disruption of blood vessels and loss of the newly formed carbon-deuterium bonds. Basic histopathological examinations also confirmed the early apoptotic effects of all the tested anticancer interferences. Additionally, ex vivo data of DNP-MRS of hyperpolarized 13C pyruvate mixed with tumor tissue homogenates confirmed that the relative production of 13C lactate to 13C pyruvate showed a significant decrease after all these treatment strategies. On the other hand, the T2-weighted anatomical images of MRI taken on day 7 of treatment showed no significant differences in the anatomical structures or sizes of the tumors.Conclusion
In this study, a higher level of deuterium accumulation trend in the tumor tissues over normal tissues were confirmed. This fact was a target in the tracking of anticancer interferences. The possibility of monitoring early treatment effects in pancreatic carcinoma by dMRI was demonstrated in 3 anticancer strategies. Radiation therapy, Bevacizumab and Gemcitabine injection proved effective in the treatment of pancreatic cancer in MIA PaCa-2 murine models using D2O labelling-dependent dMRI. Overall, the present research indicates a promising clinical usage of the dMRI, a minimally invasive approach, for monitoring the treatment of cancer patients and offers new ways of studying and characterizing tumor progression and treatment. This straightforward, non-radioactive imaging technique might also be a helpful addition to the imaging toolset for cancer theranostics.Summary of Main Findings
Using D2O labeling followed by dMRI using 1.5T MRI, we imaged mice tissues. Three anticancer therapies proved gradual and significant reductions in deuterium signals along 7 days of imaging in a murine model of human pancreatic cancer.Acknowledgements
No acknowledgement found.References
1. Lu M, Zhu XH, Zhang Y, Mateescu G, Chen W. Quantitative assessment of brain glucose metabolic rates using in vivo deuterium magnetic resonance spectroscopy. (1559-7016 (Electronic)).
2. De Feyter HM, Behar KL, Corbin ZA, Fulbright RK, Brown PB, McIntyre S, et al. Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo. Science advances. 2018;4(8):eaat7314. Epub 2018/08/25.
3. Kreis F, Wright AJ, Hesse F, Fala M, Hu D-e, Brindle KM. Measuring Tumor Glycolytic Flux in Vivo by Using Fast Deuterium MRI. Radiology. 2020;294(2):289-96.
4. Li H, Zhu X-H, Zhu W, Lee B-Y, Wiesner HM, Zhang Y, et al., editors. Dynamic Deuterium MRS Imaging for Studying Rat Heart Energy Metabolism In Vivo—Initial Experience. Proc Intl Soc Mag Reson Med; 2019.
5. Ruhm L, Avdievich N, Ziegs T, Nagel AM, De Feyter HM, de Graaf RA, et al. Deuterium metabolic imaging in the human brain at 9.4 Tesla with high spatial and temporal resolution. NeuroImage. 2021;244:118639. Epub 2021/10/13.
Figures