1487
Assessment of IVIM-DKI for characterization of benign and malignant lymph nodes in lymphoma
Archana Vadiraj Malagi1,2, Devasenathipathy Kandasamy3, Deepam Pushpam4, Kedar Khare5, Raju Sharma3, Rakesh Kumar6, Sameer Bakhshi4, and Amit Mehndiratta1,7
1Centre for Biomedical Engineering, Indian Institute of Technology Delhi (IIT Delhi), New Delhi, India, 2Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 3Department of Radiodiagnosis, All India Institute of Medical Sciences Delhi, New Delhi, India, 4Department of Medical Oncology, Dr. B.R. Ambedkar Institute-Rotary Cancer Hospital (IRCH), All India Institute of Medical Sciences Delhi, New Delhi, India, 5Department of Physics, Indian Institute of Technology Delhi (IIT Delhi), New Delhi, India, 6Department of Nuclear Medicine, All India Institute of Medical Sciences Delhi, New Delhi, India, 7Department of Biomedical Engineering, All India Institute of Medical Sciences Delhi, New Delhi, India
1Centre for Biomedical Engineering, Indian Institute of Technology Delhi (IIT Delhi), New Delhi, India, 2Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 3Department of Radiodiagnosis, All India Institute of Medical Sciences Delhi, New Delhi, India, 4Department of Medical Oncology, Dr. B.R. Ambedkar Institute-Rotary Cancer Hospital (IRCH), All India Institute of Medical Sciences Delhi, New Delhi, India, 5Department of Physics, Indian Institute of Technology Delhi (IIT Delhi), New Delhi, India, 6Department of Nuclear Medicine, All India Institute of Medical Sciences Delhi, New Delhi, India, 7Department of Biomedical Engineering, All India Institute of Medical Sciences Delhi, New Delhi, India
Synopsis
Keywords: Cancer, Diffusion/other diffusion imaging techniques
Intravoxel Incoherent motion-diffusion kurtosis imaging (IVIM-DKI) was used for evaluation and characterization of malignant lymph nodes in lymphoma. A total of twenty-one (n=21) patients diagnosed with biopsy proven Hodgkin lymphoma(HL: n=13) or non-Hodgkin lymphoma(NHL: n=8) were prospectively evaluated. IVIM-DKI parameters were estimated using standard IVIM-DKI model (standard model) and IVIM-DKI model with total variation(TV) penalty function method(IDTV model). Perfusion fraction (f) and kurtosis (k) estimated using IDTV model, and apparent diffusion coefficient(ADC) were significantly(p<0.05) lower in malignant lymph nodes than benign lymph nodes. f and k showed high AUC of 0.88 and 0.83, respectively for malignant vs. benign lymph nodes.Introduction
Intravoxel incoherent motion (IVIM) and diffusion kurtosis imaging (DKI) analysis in oncological imaging have shown promising results in detection, characterization, evaluation, and the prediction of treatment response for various cancers1–3. A non-Gaussian model of water diffusion has been shown to accurately detect tumor heterogeneity such as IVIM with DKI (IVIM-DKI)4. There is limited literature on the characterization of benign and malignant lymph nodes in Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL) utilizing IVIM-DKI, mostly on head and neck cancer4,5. Thus, this study aimed to investigate the role of quantitative parameters such as diffusion coefficient (D), pseudo-diffusion coefficient (D*), perfusion fraction (f), and kurtosis (k) obtained from the multi-b-values IVIM-DKI model with the TV method6–8 for the characterization of benign and malignant lymph nodes in lymphoma.Methods
Subjects: A total of 21 patients were included in the analysis under this investigation (Age (mean±SD):34±11.4 years old, Female/Male:4/17). As indicated in figure 1, histopathological results revealed three different subsets of lymphoma with HL (n=13) and NHL comprising of Diffuse large B-cell lymphoma (DLBCL:n=5), and follicular lymphoma (n=3).ROI localization: The whole volume ROI was drawn manually. Figure 2(a-l) demonstrates the ROI placement for malignant lymph nodes in HL and NHL. Benign lymph node ROI was drawn on IVIM-DKI dataset at b-values=0 s/mm2 where small lymph nodes displayed hyperintensity area and SUV map indicated no FDG uptake.
IVIM-DKI acquisition and Analysis: All patients were scanned in 1.5T MRI (Ingenia; Philips Healthcare, Netherlands) with a STIR (Thoracic:TR=1.503s and TE=0.09s; Abdomen: TR=1.503s and TE=0.06s), including IVIM-DKI with 9b-values= 0,35,50,100,175,300,500,1500,2000 s/mm2 using phased-array surface coil (Thoracic:TR=12.44s,TE=0.081s; Abdomen: TR=12.44s,TE=0.081s) for thoracic and abdominal area. The ADC of each ROI was estimated voxelwise using a monoexponential model with the three different b-values (0,500,1500 s/mm2).
The IVIM-DKI model for multi-b-values:
$$ \frac{S I}{S I_0}=f e^{-b D^*}+(1-f) e^{-b D+\frac{1}{6} b^2 D^2 k} -(1) $$
where, SI and SI0 are diffusion signals with and without a diffusion gradient b in s/mm2. The IVIM-DKI images was analyzed using two models: (1) standard model as shown in equation 1; and (2) IDTV model, which is an IVIM-DKI model with the TV penalty function method which minimizing the total variation of a whole 3D-parametric map6–8.
Statistics: Coefficient of variation (CV) was calculated to compare the model performance of standard and IDTV models. Kolmogorov-Smirnov test was used to calculate any significant differences between the IVIM-DKI parameters of benign and malignant lymph nodes in lymphoma. Diagnostic performance of IVIM-DKI parameters were measured using receiver operating characteristics (ROC) analysis.
Results
Qualitative and Quantitative characterization of benign and malignant lymph nodes in lymphoma using IVIM-DKIFigure 3 shows a representative image of a 32 year old male patient with DLBCL, stage IV, with a tumor in the anterior chest wall. In the tumor region, a high b-value map, and STIR image showed a hyperintense region, as shown in Figure 3. IDTV model showed significantly (p<0.001) lower CV than the standard model by 42-59% in both benign and malignant lymph nodes for all IVIM-DKI parameters. ADC and IVIM-DKI parameters estimated using the IDTV model were evaluated to characterize benign and malignant lymph nodes. Figure 4(a, d, e) shows that ADC, f, and k values were significantly (p<0.05) lower in malignant lymph nodes than in benign lymph nodes in lymphoma. D and D* did not show significant differences in the benign and malignant lymph nodes.
Differential diagnosis of benign and malignant lymph nodes using IVIM-DKI and ROC analysis
High diagnostic performance was obtained for the f parameter, which showed the highest area under curve (AUC), accuracy, and F1 score of 0.88, 86%, and 86%, with a cut-off value of 0.223 to differentiate between nodes, as shown in figure 5. Even the k parameter showed high diagnostic performance, with AUC, accuracy, and F1 score of 0.83, 81%, and 79%, with a cut-off value of 0.993.
Discussion and Conclusion
In the present study, benign and malignant lymph nodes in lymphoma were characterized using ADC and IVIM-DKI parameters. In benign lymph nodes, ADC was higher than in malignant lymph nodes in the lymphoma. ADC is measured using a conventional monoexponential function and has shown its potential in the differentiation of benign and malignant lymph nodes3,9. However, IVIM-DKI can produce quantitative diffusion and perfusion information with additional kurtosis information on tumor heterogeneity at low b-values (100 s/mm2) and high b-values (>1500 s/mm2)4,10,11. In this study, a parametric reconstruction method such as TV was employed with the IVIM-DKI model, which has been shown to remove any spurious values while estimating parameters and improve the characterization of prostatic lesions6. f and k estimated using the IDTV model were able to differentiate benign and malignant lymph nodes, same as also seen in previous studies12. f represents blood volume fraction, and high perfusion in benign nodes may indicate the presence of vasodilation, increased blood flow, and high permeability13. However, k was lower in malignant than benign lymph nodes due to the presence of inflammation or fibrous tissues14. While f and k showed higher AUC and accuracy compared to ADC in differentiation between benign and malignant lymph nodes. This demonstrates that the IDTV model may provide high diagnostic performance even when parameters are employed individually.Acknowledgements
The authors would like to thank SERB, the Department of Science and Technology, the Government of India (CRG/2021/005342) for the funding support.References
- Wu, R. et al. Assessment of chemotherapy response in non-Hodgkin lymphoma involving the neck utilizing diffusion kurtosis imaging: a preliminary study. Diagnostic and Interventional Radiology 23, 245–249 (2017).
- Beyhan, M., Sade, R., Koc, E., Adanur, S. & Kantarci, M. The evaluation of prostate lesions with IVIM DWI and MR perfusion parameters at 3T MRI. La radiologia medica 124, 87–93 (2019).
- Lecler, A. et al. Intravoxel incoherent motion (IVIM) 3 T MRI for orbital lesion characterization. Eur Radiol 31, 14–23 (2021).
- Lu, Y. et al. Extension of the intravoxel incoherent motion model to non-gaussian diffusion in head and neck cancer. Journal of Magnetic Resonance Imaging 36, 1088–1096 (2012).
- Sijtsema, N. D. et al. An optimal acquisition and post‐processing pipeline for hybrid IVIM‐DKI in head and neck. Magnetic Resonance in Medicine (2020) doi:https://doi.org/10.1002/mrm.28461.
- Malagi, A. V. et al. IVIM–DKI for differentiation between prostate cancer and benign prostatic hyperplasia: comparison of 1.5 T vs. 3 T MRI. Magnetic Resonance Materials in Physics, Biology and Medicine (2021) doi:10.1007/s10334-021-00932-1.
- Kayal, E. B. et al. Quantitative Analysis of Intravoxel Incoherent Motion (IVIM) Diffusion MRI using Total Variation and Huber Penalty Function. Medical Physics 44, 5849–5858 (2017).
- Rudin, L. I., Osher, S. & Fatemi, E. Nonlinear total variation based noise removal algorithms. Physica D: Nonlinear Phenomena 60, 259–268 (1992).
- De Paepe, K. N. et al. Improving lymph node characterization in staging malignant lymphoma using first-order ADC texture analysis from whole-body diffusion-weighted MRI. Journal of Magnetic Resonance Imaging 48, 897–906 (2018).
- Le Bihan, D. et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168, 497–505 (1988).
- Jensen, J. H., Helpern, J. A., Ramani, A., Lu, H. & Kaczynski, K. Diffusional kurtosis imaging: The quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magnetic Resonance in Medicine 53, 1432–1440 (2005).
- Yu, J. et al. Diffusion kurtosis imaging in identifying the malignancy of lymph nodes during the primary staging of rectal cancer. Colorectal Disease 20, 116–125 (2018).
- Xu, C. et al. Value of integrated PET-IVIM MR in assessing metastases in hypermetabolic pelvic lymph nodes in cervical cancer: a multi-parameter study. Eur Radiol 30, 2483–2492 (2020).
- Liu, Y. et al. The diagnostic accuracy of intravoxel incoherent motion and diffusion kurtosis imaging in the differentiation of malignant and benign soft-tissue masses: which is better? Acta Radiol 63, 785–793 (2022).
Figures
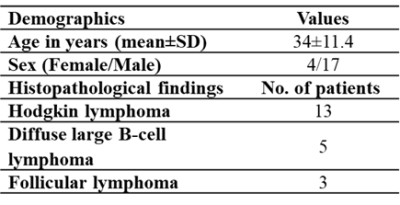
Figure 1: Demographics of patients
suffering from lymphoma with histopathological information
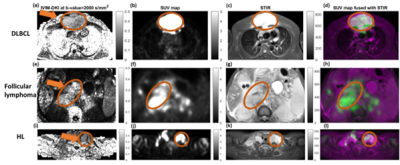
Figure 2: Representative images of
(a-d) of 32 year old male patient suffering from Diffuse large B-cell lymphoma
(DLBCL) at stage IV, (e-h) 45 year old male patient suffering from follicular
lymphoma at stage III, and (i-l) 28 year old female patient suffering from
classical Hodgkin lymphoma (HL), Nodular Sclerosis type at stage IV, showing
hyperintensity in tumor region (encircled in white color) in (a,e,i) IVIM-DKI
at 2000 s/mm2, (b,f,j) SUV map, (c,g,k) STIR image, and (d,h,l)
images showing fusion between SUV and STIR image.
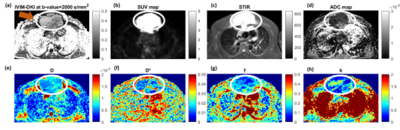
Figure 3:
Representative images of 32 year old male patient suffering from Diffuse large
B-cell lymphoma (DLBCL) at stage IV showing (a) IVIM-DKI at 2000 s/mm2,
(b) SUV map, (c) STIR images, and (d)
ADC map. (e) D, (f, j) D*, (g, k) f, and (h, l) k maps. In tumor region (white
encircled), hyperintensity was observed on (a) IVIM-DKI at 2000 s/mm2,
(b) SUV map, (c) STIR images, (f) D*
map, and (h) k map and hypointensity was observed on (d) ADC map, (e) D map,
and (g) f map.
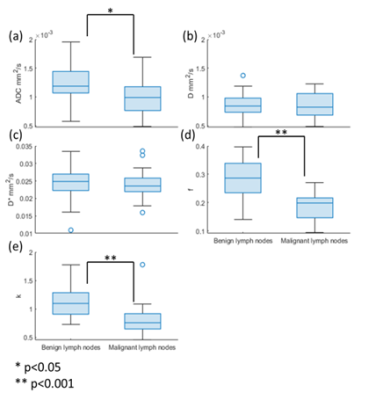
Figure 4: Boxplot showing comparison
between ADC and IVIM-DKI parameters quantified from IDTV model in benign and
malignant lymph nodes. ADC, f, and k were significantly (p<0.05) lower in
malignant lymph node than benign lymph node.
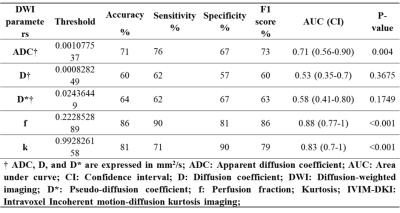
Figure 5:
Characterization of benign and malignant lymph nodes in the lymphoma using ADC and IVIM-DKI parameters estimated with IDTV model
DOI: https://doi.org/10.58530/2023/1487