1484
Multi-parametric MRI-based Peritumoral Radiomics for Stage IIA and IIB Classification of Cervical Cancer:A Multicenter Study1The First Affiliated Hospital of Jinan University, Guangzhou, China, 2Neusoft Medical Systems Co, Shanghai, China
Synopsis
Keywords: Uterus, Radiomics
Appropriate management and treatment decisions for cervical cancer depend on accurate staging, but the differentiation between IIA and IIB by imaging assessment remains difficult. This retrospective study investigated the performance of intratumoral and peritumoral multi-parameter MRI texture information in differentiating IIA and IIB using non-invasive radiomics analysis, which was also compared with clinical factors and imaging assessment by radiologists. Among all the comparisons, peritumoral-based radiomics models outperformed the radiologists and performed the best. This study offers a viable approach in non-invasively and accurately differentiating IIA and IIB for cervical cancer based on peritumoral texture information.Appropriate management and treatment decisions for cervical cancer depends on accurate staging. Surgery is the first-line treatment for early-stage cervical cancer (IA-IIA), while concurrent chemoradiothrapy (CCRT) is the standard treatment in locally advanced cervical cancer (IIB-IV).1 According to the new 2018 FIGO staging, clinicians usually use preoperative MRI and biopsy to assist the diagnosis and staging of cervical cancer.2 However, histopathological information can only be obtained through invasive methods. The excisional biopsy often disrupts the original microenvironment of the tumor and compromises further pathological findings.3 Meanwhile, imaging assessments performed by radiologists heavily rely on their clinical experience, resulting in large subjective variations. Especially, the differentiation of stages IIA and IIB are nearly unachievable through MRI images4, which might be addressed by employing radiomics. Radiomics can extract high throughput image features that shows great potential in diagnosis, response to therapy and prognosis.5 In this retrospective study, we aimed to explore intratumoral and peritumoral multi-parameter MRI-based radiomics to establish imaging-based classifiers for distinguishing stage IIA vs. stage IIB of cervical cancer.
Methods
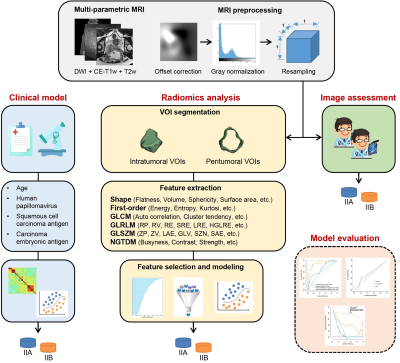
Two hundred and eight cases with histologically confirmed cervical cancer from three institutions (Hospital 1: n = 67; Hospital 2: n = 36; Hospital 3: n = 105) were enrolled in this study. All the cases were randomly divided into the training cohort (n=145) and the validation cohort (n=63). The design of this study is shown in Figure 1. Four clinical factors including age, human papillomavirus, squamous cell carcinoma antigen, and carcinoma embryonic antigen were collected. Univariate and multivariate logistic regression analyses were respectively applied to these factors for the clinical model development. For the multi-parametric MRI acquisition, diffusion-weighted imaging (DWI), contrast-enhanced T1-weighted, and T2-weighted imaging were performed. MRI standardization processing including offset correction, gray level normalization, and resampling of voxels were used to reduce the gray difference among different manufacturers, imaging protocol, and patients. Two radiologists (6 and 10 years of experience, respectively) independently and double-blindly determined the clinical stage from MRI and segmented the volume of interest (VOI). The final version was obtained through discussion and corrected for the cases with large differences in the definitions from the radiologists. Peritumoral VOIs were defined from the final VOIs dilated by a circular structural element with a radius size (number of a pixel) of 3. An open-source package PyRadiomics6 was used for radiomics feature extraction. Then, a total of 1130 radiomics features were extracted from each VOI of the original and derived MR images using the Laplacian of gaussian and wavelet filter. After the feature standardization with Z-score algorithm, the Pearson correlation coefficient analysis and recursive feature elimination algorithm were adopted successively to obtain the well-representative features. Different classifiers were compared to develop the optimal radiomics signature across 5-fold cross validation. The calibration curves and decision curve analysis were conducted to evaluate the clinical utility of the optimal model.
Results
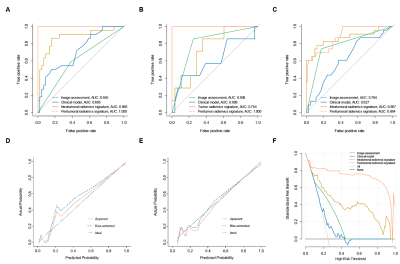
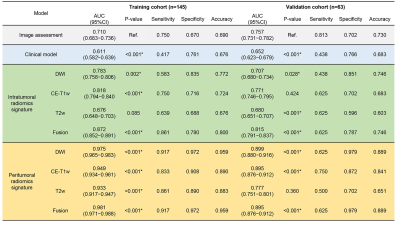
The comparison results of the predictive models are shown in Table 1. Only age (odds ratio, 0.962; 95% confidence interval, 0.925−0.999; P=0.047*) was found to be significantly associated with the stage and used to construct the clinical model. The peritumoral radiomics models were superior to the intratumoral radiomics models, regardless of single sequence model or fusion model (all P <0.001*). DWI-based peritumoral radiomics model performed best with the AUCs of 0.975 (0.965−0.983) and 0.899 (0.880−0.916) in the training and validation cohort, respectively. There was no significant difference between the validation AUCs of DWI-based and fusion peritumoral radiomics model (0.899 vs. 0.895, P =0.566). Figure 2 illustrates the superior performance of this model.
Discussion
Distinguishing IIA and IIB stages is essential because the therapeutic regimens and follow-up care are vastly different for each of these conditions. We explored the intratumoral and peritumoral regions of cervical cancer in differentiating IIA and IIB stages, and the results revealed that the most predictable features could be obtained from the peritumoral regions with 3 pixel dilation distances in the DWI images. Parametrial involvement (PMI) is the main differentiation between IIA and IIB stages. Previous studies showed that DWI is regarded as the most efficient tool to be employed in the detection of PMI. The results can be interpreted considering that DWI images can detect early pathological changes associated with changes in water content in tissues and can provide good functional information7,8. Similar to our previous finding, this study suggested that the peritumoral radiomic signatures have a much better discrimination performance in distinguishing IIA and IIB stage, which can be partly explained by that the fact that peritumoral region provides more information about parametrial infiltration 9,10.
Conclusion
MRI-based radiomics model from peritumoral regions outperformed radiologists for the preoperative diagnosis of IIA and IIB stage. A noninvasive and reliable supplementary approach was provided to precise preoperative staging, which can help optimize individualized treatment plans for patients with cervical cancer.
Acknowledgements
No acknowledgement found.References
Reference
1. Cohen PA, Jhingran A, Oaknin A, et al. Cervical cancer. Lancet. 2019;393(10167):169-182. 2. Bhatla N, Berek JS, Cuello Fredes M, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet. 2019;145(1):129-135.
3. Weyl A, Illac C, Lusque A, et al. Prognostic value of lymphovascular space invasion in early-stage cervical cancer. Int J Gynecol Cancer. 2020;30(10):1493-1499.
4. Manganaro L, Lakhman Y, Bharwani N, et al. Correction to: Staging, recurrence and follow-up of uterine cervical cancer using MRI: Updated Guidelines of the European Society of Urogenital Radiology after revised FIGO staging 2018. Eur Radiol. 2022;32(1):738.
5. O'Connor JP, Rose CJ, Waterton JC, et al. Imaging intratumor heterogeneity: role in therapy response, resistance, and clinical outcome. Clin Cancer Res. 2015;21(2):249-257.
6. van Griethuysen J, Fedorov A, Parmar C, et al. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017; 77(21):e104-e107.
7. Park JJ, Kim CK, Park SY, et al. Value of diffusion-weighted imaging in predicting parametrial invasion in stage IA2-IIA cervical cancer. Eur Radiol. 2014;24(5):1081-1088.
8. Woo S, Suh CH, Kim SY, et al. Magnetic resonance imaging for detection of parametrial invasion in cervical cancer: An updated systematic review and meta-analysis of the literature between 2012 and 2016. Eur Radiol. 2018;28(2):530-541.
9. Wang X, Zhao X, Li Q, et al. Can peritumoral radiomics increase the efficiency of the prediction for lymph node metastasis in clinical stage T1 lung adenocarcinoma on CT? Eur Radiol. 2019;29(11):6049-6058.
10. Sun C, Tian X, Liu Z, et al. Radiomic analysis for pretreatment prediction of response to neoadjuvant chemotherapy in locally advanced cervical cancer: A multicentre study. EBioMedicine. 2019;46:160-169.
Figures