1479
Multi-parametric radiomics of MRI for preoperative assessment of microvascular invasion and prognosis of hepatocellular carcinoma
Lili Wang1, Junqiang Lei1, Junfeng Li2, Shunlin Guo2, Gang Wang2, and Rui Wang3
1Radiology, First Hospital of Lanzhou University, Lanzhou, China, 2First Hospital of Lanzhou University, Lanzhou, China, 3First Clinical Medical School of Lanzhou University, Lanzhou, Chile
1Radiology, First Hospital of Lanzhou University, Lanzhou, China, 2First Hospital of Lanzhou University, Lanzhou, China, 3First Clinical Medical School of Lanzhou University, Lanzhou, Chile
Synopsis
Keywords: Liver, Radiomics
To preoperatively predict MVI in HCC patients, this study developed and validated an MVI nomogram prediction model that reveals the features derived from tumor and peritumor tissue of different sequences in Gd-EOB-DTPA dynamic contrast-enhanced MR and combines the clinical and radiological signatures. The nomogram included AFP, capsule appearance, arterial peritumoral enhancement, RVI, and radiomics score, which were independent risk factors for MVI. Finally, this nomogram model achieved satisfactory performance in predicting MVI in both the training and validation cohorts. Moreover, the RFS of the nomogram model was similar to the histopathology outcome.Introduction
Microvascular invasion (MVI) is a predictor of recurrence and overall survival in hepatocellular carcinoma (HCC), the preoperative diagnosis of MVI through noninvasive methods play an important role in clinical treatment1,2. The aim of this study was to investigate the effectiveness of radiomics features in evaluating MVI in HCC before surgery.Methods
We enrolled 190 patients who had undergone dynamic contrast-enhanced MRI and curative resection for HCC between September 2015 and November 2021 from two independent institutions (Figure 1). In the training cohort of 117 patients, MVI-related radiomics models based on multiple sequences and multiple regions from MRI were constructed. An independent cohort of 73 patients was used to validate the proposed models. A final Clinical–Imaging–Radiomics nomogram for preoperatively predicting MVI in HCC patients was generated (Figure 2). Recurrence-free survival was analyzed using the log-rank test.Results
Pathological examination confirmed MVI in 78 of the 190 patients (41.05%). For tumor-extracted features, the performance of signatures in fat-suppressed T1-weighted images (T1WI-FS) and hepatobiliary phase (HBP) was superior to that of other sequences in a single-sequence model. The radiomics signatures demonstrated better discriminatory ability than that of the Clinical–Imaging model for MVI in the training (AUC = 0.842 vs. 0.747) and validation cohorts (AUC = 0.804 vs. 0.722). The nomogram incorporating alpha-fetoprotein (AFP), capsule appearance, arterial peritumoral enhancement, radiogenomic invasion (RVI), and radiomics signature showed excellent predictive ability (AUC = 0.891 vs. 0.836, training and validation cohorts, respectively) and achieved well-fitted calibration curves, outperforming both the Radiomics and Clinical–Radiomics models in the training and validation cohorts (Figure 3).Conclusion
The Clinical–Imaging–Radiomics nomogram model of multiple regions and multiple sequences based on serum AFP, three MRI characteristics, and 12 radiomics signatures achieved good performance for predicting MVI in HCC patients, which may help clinicians select optimal treatment strategies to improve subsequent clinical outcomes (Figure 4).Acknowledgements
This work was supported by funds from the First Hospital of Lanzhou University (grant number: ldyyyn2020-14), National Natural Science Foundation (grant number: 81800528), National Natural Science Foundation (grant number: 81960323). The authors thank all patients involved in the study.References
1. Dhir M, Melin AA, Douaiher J, et al. A Review and Update of Treatment Options and Controversies in the Management of Hepatocellular Carcinoma. Ann Surg 2016; 263(6): 1112-1125. https://doi.org/10.1097/sla.0000000000001556
2. Hirokawa F, Hayashi M, Miyamoto Y, et al. Outcomes and predictors of microvascular invasion of solitary hepatocellular carcinoma. Hepatol Res 2014; 44(8): 846-853. https://doi.org/10.1111/hepr.12196
Figures
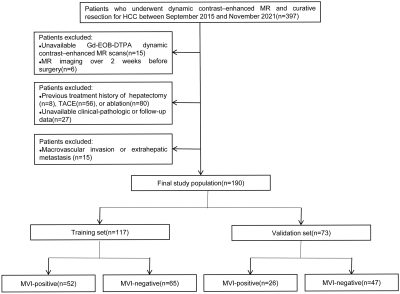
Figure 1. Flow
chart of the enrolled study patients.
HCC:
hepatocellular carcinoma; TACE: transcatheter arterial chemoembolization; MVI:
microvascular invasion.
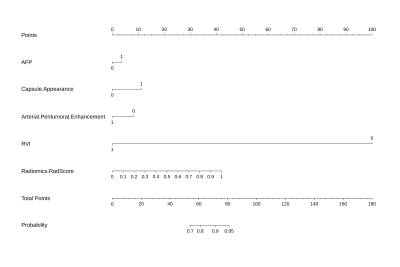
Figure 2. Nomograms for predicting MVI. The final predictive
model of MVI was visualized as nomograms.
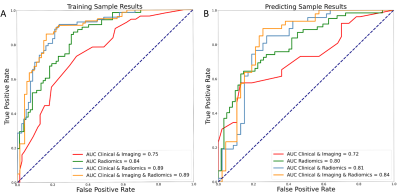
Figure 3. Comparison
ROC curves of different models for the prediction of MVI. Including ROC curves
of Radiomics model, Clinical-Imaging model, Clinical- Radiomics model,
Clinical-Imaging-Radiomics model in the training (A) and validation (B)
datasets. ROC: receiver operating characteristic; AUC: area under the receiver
operating characteristic.
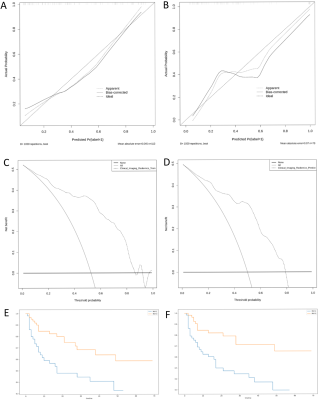
Figure
4. Calibration curve of the nomogram model in (A)
training cohort and (B) validation cohort. The dotted line represents the
ideal prediction, and the solid line represents the predictive performance of
the nomogram, and the diagonal dashed line indicates the ideal prediction by a
perfect model. Decision
curve analysis (DCA) for the nomogram in (C) training cohort and (D) validation
cohort. The dotted line represents the nomogram. Recurrence-free survival (RFS) curves were
scaled by histologic MVI status (E) and final predicted MVI model (F) by Kaplan
Meier analysis.
DOI: https://doi.org/10.58530/2023/1479