1466
Imaging water exchange in the choroid plexus using T2-prepared long TE Fluid-Attenuated Inversion Recovery1Division of MRI Research, department of Radiology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States
Synopsis
Keywords: Neurofluids, Brain
The choroid plexus (CP) plays a key role in brain homeostasis and waste clearance as the main source of CSF production in the brain. However, this small structure sitting in the lateral ventricles is not well characterized, including its dysfunction or impairment in several pathologies or just normal aging. Water exchange measurement using various methods has been proposed to evaluate structural but also functional properties of the CP such as ASL or other spin labeling strategies mainly in rodents. In this work, we propose a new look into water exchange between CP and CSF using T2-prepared, long TE Fluid-Attenuated-Inversion-Recovery (FLAIR).Introduction
The choroid plexus (CP) plays a key role in brain homeostasis and waste clearance as the main source of cerebrospinal fluid (CSF) production in the brain. However, this small structure sitting in the lateral ventricles is not well characterized, including its dysfunction or impairment in several pathologies or just normal aging.Water exchange measurement using various MRI methods has been proposed to evaluate structural but also functional properties of tissues, using methods relying on some variants of spin labeling. For example Arterial Spin Labeling has been used to probe CP function by measuring its blood-flow1 but also blood-CSF exchange2,3. Additionally, the potential for spin labeling the CP to observe exchange with the CSF has been explored in rodents4,5. In this work, we propose a new look into water exchange between choroid plexus and CSF using T2-prepared, long TE Fluid-Attenuated-Inversion-Recovery (FLAIR).Methods
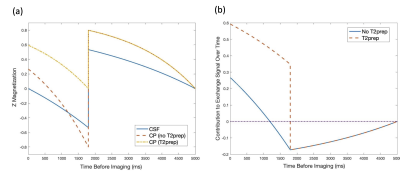
Theory: In the absence of exchange, the CSF signal is nulled by inversion timing and the CP signal is effectively nulled by T2 decay over the very long TE. However, magnetization that begins in the CP and exchanges to the CSF during the preparation time will not be fully nulled. If we approximate exchange as an immediate exchange of spins between the CSF and CP without any return, then the exchange signal will be the integral over time before imaging of the difference between CP and CSF magnetization times the T1 decay factor of CSF before imaging. The IR pulse effectively inverts the contribution before the pulse. When a T2-preparation pulse is applied before the inversion pulse, shorter T2 tissues including the CP are essentially nulled. The magnetization as a function of time before imaging is shown in Figure 1a. We also plot in Figure 1b the difference between the CP and CSF magnetization times the T1 decay factor (with the difference reversed prior to the inversion pulse). It is apparent that without T2-preparation, the exchange contribution after the inversion pulse approximately cancels the contribution from before. However, when T2-preparation is applied, the positive contribution after the inversion pulse greatly increases resulting in a large net positive exchange signal.Experiments: Scans were performed at 3T (GE Signa Premier) using a 48-ch head coil. Six healthy volunteers (44±18yo) were recruited. We acquired 3D-FSE T2-FLAIR data (TI/TR/TE=1785/6000/107ms, ETL=220, linear view-ordering) as well as a pair of long TE FLAIR acquisitions with and without T2-preparation using reversed-centric view-ordering, with 25 discarded echoes to avoid propagation of high-frequency features during early echoes, ETL=245, TR/TE=6/1s, first refocusing flip angle of 120 degrees followed by a gradual ramp down to 75 degrees6. TI was automatically adjusted on a case-by-case depending on echo-train duration with a null target of T1=4.27s taking into account the T2-preparation (TI≈1737-1770ms) as illustrated in Figure 2. An additional reference volume without IR or T2 preparation was acquired to serve as reference.The T2-preparation consisted of a 90 degrees hard pulse, followed by 4 adiabatic hyperbolic-secant refocusing pulses and one final -90 degrees hard pulse. We also acquired a separate FLAIR volume without T2-preparation and adjusted TI for CSF nulling without T2-preparation (TI≈1850-1890ms). Common parameters were: 136 sagittal slices, matrix=192x192 leading to (1.3mm)3 resolution, parallel imaging with 2x2 acceleration in both phase-encoded directions for an acquisition time of 3min21s per volume (total scan time 16.5min).
Analysis: We reconstructed real-valued volumes offline to avoid errors associated with magnitude reconstruction, followed by subtraction between T2-prepared and control volumes. We calculated an exchange signal fraction ESF=T2Pon(CP)-T2Poff(CP)/Ref(CSF) with CP and CSF corresponding to the mean value in a ROI positioned in the choroid plexus and neighboring CSF respectively.
Results
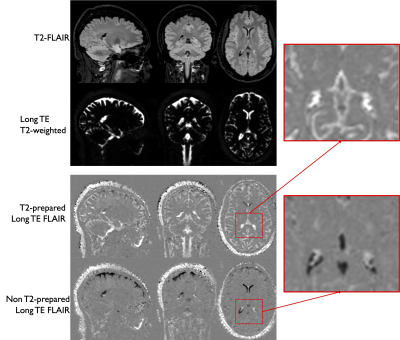
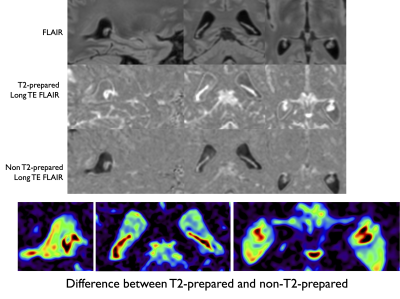
Representative images are shown in Figure 3. We can see that the signal in the CP is substantially higher in the T2-prepared volume compared to the control. Figure 4 shows another example showing again the higher signal in the T2-prepared volume, with in this case a subtraction between T2-prepared and control volumes showing higher signal in the CP compared to surrounding CSF. The subtraction experiment allows controlling for potential remaining T2 signal in the CP, but the imperfect CSF nulling in the control volume leads to substantial CSF contamination in the subtraction image. The average ESF was found equal to 2.2±0.4%.In one volunteer, we also acquired T2-prepared data with significantly longer TE of 1.8s to eliminate any possibility of residual CP signal contamination. The presence of a strong remaining signal in the CP at such TE confirms water exchange as the source of the strong signal in the CP.Discussion and Conclusions
We have highlighted evidence of water exchange in the choroid plexus using T2-prepared, long TE FLAIR imaging. Although the full exchange mechanism and model remains to be fully elucidated, this work produced high-resolution images of the CP and could open new possibilities for studying CP function, complementary to other methods such as ASL that has recently gained traction for the study of CP function. Future work will focus on providing a robust control that adequately matches CSF signal levels to the T2-prepared acquisition in order to support quantification. This could be evaluated in normal populations but also in pathology such as Alzheimer’s disease.Acknowledgements
No acknowledgement found.References
1. Zhao, L., Taso, M., Dai, W., Press, D. Z. & Alsop, D. C. Non-invasive measurement of choroid plexus apparent blood flow with arterial spin labeling. Fluids and Barriers of the CNS 17, 58 (2020).
2. Evans, P. G. et al. Non-Invasive MRI of Blood–Cerebrospinal Fluid Barrier Function. Nat Commun 11, 2081 (2020).
3. Petitclerc, L. et al. Ultra-long-TE arterial spin labeling reveals rapid and brain-wide blood-to-CSF water transport in humans. NeuroImage 245, 118755 (2021).
4. Li, A. M. & Xu, J. Cerebrospinal fluid-tissue exchange revealed by phase alternate labeling with null recovery MRI. Magnetic Resonance in Medicine 87, 1207–1217 (2022).
5. Li, A. M. et al. Age-dependent cerebrospinal fluid-tissue water exchange detected by magnetization transfer indirect spin labeling MRI. Magnetic Resonance in Medicine 87, 2287–2298 (2022).
6. Alsop, D. C. The sensitivity of low flip angle RARE imaging. Magnetic Resonance in Medicine 37, 176–184 (1997).
Figures