1463
Measurement of CSF pulsation in Parkinson’s disease patients using EPI-based fMRI data1Korea Advanced Institute of Science and Technology, Daejeon, Korea, Republic of, 2Yonsei University Colledge of Medicine, Seoul, Korea, Republic of
Synopsis
Keywords: Neurofluids, Parkinson's Disease
The fMRI dataset for Parkinson’s disease with mild cognitive impairment (PD-MCI) was studied using a recently-proposed method of simultaneous CSF pulsation and BOLD activity imaging. The PD group was classified to dementia (PDD) high-risk and low-risk groups. The CSF pulsation of both PDD high-risk and low-risk groups was higher than that of healthy control. Opposite to the tendency of CSF pulsation, coefficient of variation of CSF pulsation was reduced in PD group compared to healthy control. These results indicate association of CSF pulsation with brain waste clearance in these patients, which requires further investigations for elucidation.Introduction
Recently, cerebrospinal fluid (CSF) flow has been highlighted for its function of brain waste clearance1. CSF flow is involved in brain glymphatic and meningeal lymphatic clearance systems by mediating the transport of metabolic waste products from the brain to the outside of cranium. Previous studies reported that impairment in brain clearance steps is associated with neurodegenerative diseases, cognitive deficit, and central nervous disorders2. There are representative neurodegenerative diseases thought to be associated with defect in CSF flow such as Alzheimer’s disease and Parkinson’s disease. Based on the previous studies, correlation between global-BOLD and CSF inflow was significantly low in both Alzheimer’s disease and Parkinson’s disease with dementia3,4. However, direct correlation with CSF pulsation and development of neurodegenerative diseases has not been studied yet. In this study, we aimed to demonstrate the relationship between CSF pulsation and cognitive deficit development of Parkinson’s disease with mild cognitive impairment (PD-MCI) dataset using a novel CSF pulsation measurement technique based on EPI-based fMRI5.Method
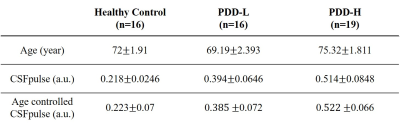
All the experiments were performed on a 3T whole-body scanner (Phillips). We used fMRI data from 17 healthy controls (HC) and 35 patients with PD-MCI. The PD-MCI patients were classified into a PD with dementia high-risk group (PDD-H, n=19) and a low-risk group (PDD-L, n=16), based on whether they developed dementia within 5 years. The ages of PDD-L patients, PDD-H patients, and HC subjects were 69.19±2.393, 75.32±1.811, and 72±1.91, respectively (Table.1).For resting-state fMRI, 2D multi-slice EPI images were acquired with following parameters: repetition time(TR)/echo time(TE)/flip angle = 2000msec/30msec/90°, resolution=2.75×2.75, slice thickness=4.2mm, matrix size=80×80, slice order=ascending interleaved, number of slices=31. Total of 160 measurements were performed for the resting-state fMRI with the whole brain coverage. EPI images were preprocessed using FSL including temporal high pass filter (0.01Hz), motion correction and slice-timing correction5.
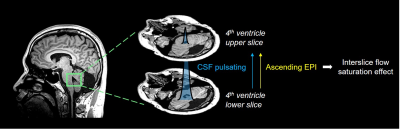
To measure CSF pulsation from fMRI data, we used interslice flow saturation effect during EPI acquisition. Based on the EPI signal simulation and CSF pulsation modeling, amount of CSF pulsation(CSFpulse) could be measured5. From the previous study, the CSFpulse was highly correlated with stroke volume measured with phase contrast MRI in aqueduct, which reflects ventricular CSF pulsation. CSF signal from the two interleaved 4th ventricle slices were used for interslice CSF signals for CSFpulse processing(Fig.1). The quantitative metric of CSF pulsation(CSFpulse) was calculated as below to represent the interslice pulsed CSF volume. $$$CSFpulse(n)=(\frac{1}{α-1})×(\frac{S_i (n)}{(S_{i-1} (n) }-1)×ROIsize$$$, where indicates ith slice CSF signal intensity in the nth measurement and indicates the ratio between the pulsating CSF signal and non-pulsated steady state CSF signal5(Fig.1).
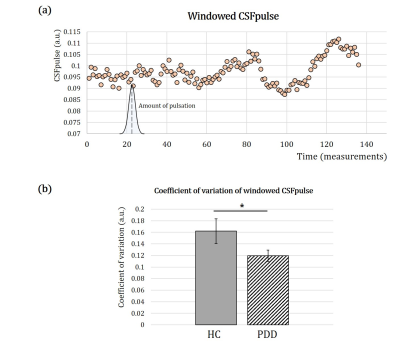
The target 4th ventricle slices were selected manually and the ROI of 4th-ventricle CSF was mapped automatically based on the intensity thresholding. Then, CSFpulse was calculated from 155 EPI measurements (excluding the first 5 measurements), where each CSFpulse indicated single pulsation amount during the scan. The dynamic CSFpulse for each subject was to represent the mean CSF pulsation during resting-state. To evaluate the variation in CSF pulsation, we acquired coefficient of variation(=std/mean) of CSFpulse from each subject after windowing 25 measurements.
We excluded extreme outliers of CSFpulse from each group, which were bigger than upper 25% quartile+3×interquartile range. There were two outliers from PDD-L group and one outlier from HC group. All the statistical tests were conducted using SPSS (version 25; IBM Corp.) and MATLAB R2020a (Mathworks). To compare the difference in CSFpulse between HC, PDD-L and PDD-H, two-sample t-test was conducted for statistical evaluation.
Result
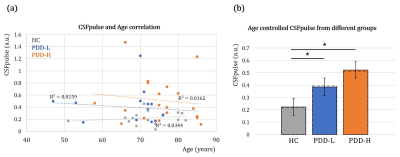
The CSFpulse values from HC group and patient groups were adjusted with age covariate(Fig.2.a). After adjusting the age covariance from CSFpulse, CSFpulse values from both PDD-H and PDD-L were significantly higher than those of the HC(p<0.05; two-sample t-test). Difference in CSFpulse between PDD-L and PDD-H was not statistically significant(p=0.292; two-sampled t-test).During the resting state, CSF pulsation amplitude could be changed. To evaluate this pulsation alteration, we checked coefficient of variation(CoV) of windowed CSFpulse. Windowing CSFpulse removed noise influence on temporal processing(Fig.3.a). The average CoV from HC group was significantly higher than CoV from PDD group(p<0.05; two-sample t-test)(Fig.3.b).
Discussion
In this study, we investigated the CSF pulsation(CSFpulse) of Parkinson’s disease patients with MCI. As a result, CSFpulse was increased in PDD group than HC, especially PDD-H which contributed more to CSF pulsation difference between HC and PDD(Fig.2). Opposite to the CSFpulse, CoV of CSFpulse during resting state was significantly lower in PDD group than HC(Fig.3). These results can be interpreted as the amount of CSF pulsation is increased with more waste clearance deposit. A previous study reported that large variation in CSF pulsation is important for waste clearance during sleep6. The CoV of CSFpulse difference in this study showed that brain clearance dysfunction might be associated with smaller variation in CSF pulsation. In addition, dementia development after PD could be affected by not only CSF pulsation amplitude and Cov of CSFpulse, but also outflow pathway through meningeal lymphatic dysfunction(which can be impaired with aging) or coupling between CSF inflow and global brain activity3,4,7.In summary, our study demonstrated that CSF pulsation could be related with dementia development after Parkinson’s disease from fMRI data. This study can be applied in brain functional study of Parkinson’s disease and its dementia development correlated with CSF clearance.
Acknowledgements
No acknowledgement found.References
[1] Louveau, Antoine, et al. "Understanding the functions and relationships of the glymphatic system and meningeal lymphatics." The Journal of clinical investigation 127.9 (2017): 3210-3219.
[2] Da Mesquita, Sandro, et al. "Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease." Nature 560.7717 (2018): 185-191.
[3] Han, Feng, et al. "Reduced coupling between cerebrospinal fluid flow and global brain activity is linked to Alzheimer disease–related pathology." PLoS biology 19.6 (2021): e3001233.
[4] Han, Feng, et al. "Decoupling of global brain activity and cerebrospinal fluid flow in Parkinson's Disease cognitive decline." Movement Disorders 36.9 (2021): 2066-2076.
[5] Kim, Jun-Hee, Jae-Geun Im, and Sung-Hong Park. "Measurement of CSF pulsation from EPI-based human fMRI." NeuroImage 257 (2022): 119293.
[6] Fultz, Nina E., et al. "Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep." Science 366.6465 (2019): 628-631.
[7] Ahn, Ji Hoon, et al. "Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid." Nature 572.7767 (2019): 62-66.
Figures