1456
DWI with dynamic b-value cycling reveals evidence of reduced suprasellar cistern neurofluid motion in Parkinson’s disease
Gabriela Pierobon Mays1, Kilian Hett1, Jarrod Eisma1, Colin D McKnight1, Jason Elenberger1, Alexander K Song1, Ciaran Considine1, Caleb Han1, Daniel O Claassen1, and Manus J Donahue1
1Vanderbilt University, Nashville, TN, United States
1Vanderbilt University, Nashville, TN, United States
Synopsis
Keywords: Neurofluids, Parkinson's Disease, Glymphatic
The goal of this study was to use a novel diffusion weighted imaging (DWI) protocol with dynamic cycling of low b-values to test fundamental hypotheses regarding neurofluid movement along inflow and egress pathways of the suprasellar cisterns in older adults with and without Parkinson’s disease (PD). Contrast consistent with reduced neurofluid motion was observed in PD relative to healthy participants, with reduced fluid movement corresponding to choroid plexus hyperemia. Modeling of DWI signal decay as a function of b-value provides quantitative neurofluid kinetics and may present a candidate non-tracer technology for quantifying neurofluid flow non-invasively in vivo in humans.Purpose
The goal of this work is to use DWI with dynamic cycling of b-values to test hypotheses regarding attenuated neurofluid movement within the suprasellar cisterns of older adults with and without Parkinson’s disease (PD). PD is a progressive neurodegenerative disorder with symptoms linked to protein aggregation and dopamine depletion. Cerebrospinal fluid (CSF), produced within the choroid plexus (ChP) complexes (1, 2), is the primary medium for clearance of waste product proteins and traverses traditional bulk and recently identified perivascular pathways (3, 4). Arterial pulsatility is believed to be fundamental to anterograde neurofluid movement (5), whereas fluid transport along the parasagittal dural spaces and cranial nerves (6, 7) has been proposed to have egress relevance. As major intracranial arterial vessels and multiple cranial nerves colocalize within the suprasellar cisterns, quantitative imaging of neurofluid motion within the suprasellar cisterns may provide information on neurofluid circulation dysfunction. We implemented a novel DWI protocol at the spatial resolution of the suprasellar cisterns with dynamic b-value cycling over the approximate kinetic range of CSF motion to quantify relationships between neurofluid motion and age, sex, and ChP function in older adults with and without proteinopathy secondary to PD.Subjects and Methods
Demographics. Adult participants with and without PD with no other history of neurological disease provided informed consent and were scanned at 3-Tesla (Philips).Experiment. 2D echo-planar-imaging DWI with common field-of-view=230x230x139mm, diffusion directions=6, slices=28, spatial resolution=1.8x1.8x4mm, TE=61ms, and TR=2539 ms were acquired with cycling of seven pseudo-randomized b-values (b-values = 0, 50, 100, 200, 300, 700, and 1000 s/mm2). To evaluate ChP perfusion, a recently reported pseudocontinuous arterial spin labeling (pCASL) approach tested for reproducibility and sensitivity to CSF flow was used (8) with TR=4550ms, TE=11ms, post-label delay=2000ms and with labeling placed proximal to the choroidal arteries. A non-contrast head MRI/MRA protocol (3D-T1, 3D-T2, 2D T2-FLAIR, and MRA) was applied for co-registration and to characterize tissue health.
Analysis. An exponential decay model was identified empirically (S(b) = ae-br) and voxel-wise fit to DWI-signal (S) as a function of b-value, yielding a voxel-wise map of decay (r) and scaling (a) values, whereby larger decay rates reflect faster motion. DWI and 3D-T1 data were registered non-linearly to a 2 mm atlas. Suprasellar cisterns were delineated and decay rates in this region extracted. ChP perfusion was quantified within the atria of the lateral ventricles using a deep learning algorithm applied to 3D-T1 and T2-FLAIR data (8, 9). Decay rates were contrasted between groups using a Student’s t-test and multiple regression analyses were performed to evaluate relationships with age, sex, cohort, and ChP perfusion. Significance criterion: two-sided p<0.05.
Results
We enrolled 27 PD (age=66±6.7 years, 74% male) and 32 healthy (age=68±8.9 years, 47% male) participants (Table 1). Fig. 1 shows the common suprasellar cistern region of interest and Fig. 2 shows representative images as a function of b-value within the dynamic sequence and corresponding decay maps (mm2/s). Decay rates were significantly higher in healthy (0.00328±0.00123 mm2/s) relative to PD (0.00256±0.0094 mm2/s) participants, which was significant on t-test (p=0.016) and multiple regression after controlling for age and sex (p=0.006). No relationship was observed for the decay rate and age (p=0.342) or sex (p=0.604). Fig. 3 shows representative imaging for a healthy and PD participant and Fig. 4 summarizes the distribution of values across cohorts. When the total cohort was considered, no relationship was observed between decay rate and ChP perfusion (n=59; p=0.369); when PD participants only were considered, an inverse correlation between ChP perfusion and decay rate was observed (p=0.011).Discussion
A novel dynamic DWI variant was applied with sensitivity to suprasellar cistern fluid motion in adults with PD and age-matched healthy controls. Significantly attenuated motion was observed in the suprasellar cisterns of PD relative to healthy participants. The extent of motion attenuation was associated with increased ChP perfusion in PD participants only. This latter finding suggests, but does not confirm, that ChP activity may be upregulated in the presence of reduced arterial pulsatility. The locus of the suprasellar cisterns for this technology is particularly intriguing given the spatial extent of this region relative to other smaller components of the proposed neurofluid circuit and the co-localization of this region with major intracranial arteries and cranial nerves, both hypothesized to have relevance to perivascular flow and fluid egress. Reduced neurofluid flow within the suprasellar cisterns in PD may reflect intracranial protein retention and this is being evaluated in ongoing work.Conclusion
Reduced fluid motion in the suprasellar cisterns of PD participants was observed using a novel DWI sequence with dynamic cycling of b-values.Acknowledgements
Funding: NIH/NIA 5R01AG062574, NIH/NINR 5R01NR015079, NIH/NCCIH 5R01AT011456References
1. Perera C, Harrison IF, Lythgoe MF, Thomas DL, Wells JA. Pharmacological MRI with Simultaneous Measurement of Cerebral Perfusion and Blood-Cerebrospinal Fluid Barrier Function using Interleaved Echo-Time Arterial Spin Labelling. NeuroImage. 2021;238:118270. doi:10.1016/j.neuroimage.2021.1182702. McKnight CD, Rouleau RM, Donahue MJ, Claassen DO. The Regulation of Cerebral Spinal Fluid Flow and Its Relevance to the Glymphatic System. Curr Neurol Neurosci Rep. 2020;20(12):58. doi:10.1007/s11910-020-01077-93. Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. The Lancet Neurology. 2018;17(11):1016-1024. doi:10.1016/S1474-4422(18)30318-14. Iliff JJ, Wang M, Liao Y, et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Sci Transl Med. 2012;4(147). doi:10.1126/scitranslmed.30037485. Agarwal N, Carare RO. Cerebral Vessels: An Overview of Anatomy, Physiology, and Role in the Drainage of Fluids and Solutes. Front Neurol. 2021;11:611485. doi:10.3389/fneur.2020.6114856. Hett K, McKnight CD, Eisma JJ, et al. Parasagittal dural space and cerebrospinal fluid (CSF) flow across the lifespan in healthy adults. Fluids Barriers CNS. 2022;19(1):24. doi:10.1186/s12987-022-00320-47. Kasi A, Liu C, Faiq M, Chan K. Glymphatic imaging and modulation of the optic nerve. Neural Regen Res. 2022;17(5):937. doi:10.4103/1673-5374.3248298. Eisma JJ, McKnight CD, Hett K, et al. Choroid plexus perfusion and bulk cerebrospinal fluid flow across the adult lifespan. J Cereb Blood Flow Metab. Published online October 6, 2022:0271678X2211291. doi:10.1177/0271678X2211291019. Zhao L, Feng X, Meyer CH, Alsop DC. Choroid Plexus Segmentation Using Optimized 3D U-Net. In: 2020 IEEE 17th International Symposium on Biomedical Imaging (ISBI). IEEE; 2020:381-384. doi:10.1109/ISBI45749.2020.9098443Figures
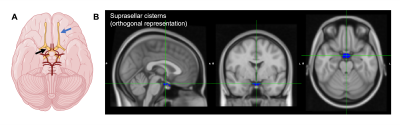
Figure 1. (A) Suprasellar cistern location, which co-localizes with major cranial nerves including the olfactory (blue arrow) and optic (black arrow), along with circle of Willis (red vasculature). (B) Regions of interest used in this study overlain on a standard 2 mm brain atlas.
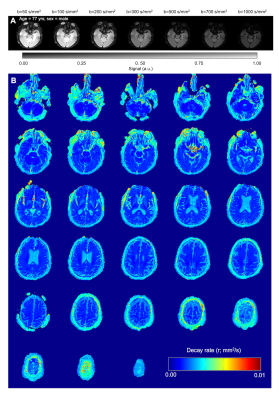
Figure 2. (A) Diffusion weighted imaging with dynamic cycling of b-values of a slice located at the level of the suprasellar cisterns for a representative participant (age=77 yrs; sex=male). (B) Corresponding quantitative decay rate maps calcualted from voxel-wise fitting of an exponential decay model to the DWI data as a function of low-to-imtermediate b-value. Warmer colors indicate faster decay and higher fluid motion.
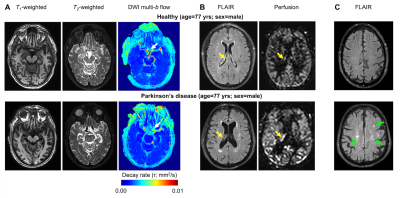
Figure 3. Case example of an age- and sex-matched healthy (above) and Parkinson’s disease (PD) (below) participant. (A) Anatomical and neurofluid flow maps at the approximate location of the suprasellar cisterns show evidence of increased motion in the healthy relative to PD participant (white arrow). (B) At the level of the lateral ventricles, evidece of hyperemia of the ChP relative to gray matter (yellow arrow) is observed in the PD participant. Group level results are summarized in Fig. 4.
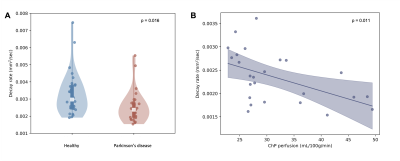
Figure 4. (A) Violin plots showing the distribution of decay rates in healthy vs. Parkinson’s disease (PD) participants, suggesting reduced neurofluid motion in PD (p=0.016). In PD participants only, reduced decay rates, indicative of slower neurofluid flow, are associated with higher choroid plexus (ChP) perfusion (p=0.011; two participants that met outlier criteria removed).
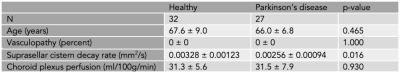
Table 1. Participant demographics and measures of suprasellar cistern motion and choroid plexus perfusion.
DOI: https://doi.org/10.58530/2023/1456