1447
Multiexponential UTE Relaxation in Achilles Tendon of Ballet Dancers Reveals Matrix Proton Pool Associated with Tendon Shear Stiffness
Anna M Horner1, Felix M Gonzalez2, Courtney N Gleason1, Amanda Blackmon3,4, Emma Faulkner3,5, and David A Reiter1
1Emory University School of Medicine, Atlanta, GA, United States, 2Radiology, AdventHealth, Orlando, FL, United States, 3Atlanta Dance Medicine, Atlanta, GA, United States, 4Physical Therapy, Mercer University, College of Health Professions, Macon, GA, United States, 5Dance, Emory University, Atlanta, GA, United States
1Emory University School of Medicine, Atlanta, GA, United States, 2Radiology, AdventHealth, Orlando, FL, United States, 3Atlanta Dance Medicine, Atlanta, GA, United States, 4Physical Therapy, Mercer University, College of Health Professions, Macon, GA, United States, 5Dance, Emory University, Atlanta, GA, United States
Synopsis
Keywords: Tendon/Ligament, Low-Field MRI, UTE MRI, SWE, ballet dancers
Multi-echo ultrashort echo time (UTE) MRI provides quantitative structural information on both tendon matrix constituents and water distribution, which influence the mechanical function of tendons. UTE images of professional ballet dancers' Achilles tendons (AT) were combined with Shear Wave Elastography (SWE) Ultrasound (US) measurements of their AT to provide insight on the structure-function. The signal fraction corresponding to the off-resonance relaxation tendon components were found to positively correlate with the long-axis SWE velocity in this dancer cohort.Introduction
Multi-echo ultrashort echo time (UTE) MRI provides noninvasive and quantitative information on both tendon matrix constituents and water distribution within the tendon matrix [1,2]. Mechanical function of tendons has been shown to be influenced by both water distribution and matrix composition [3]. We have previously assigned proton chemical shift components to tendon extracellular matrix components like collagen and proteoglycan in ex vivo tendon samples using high-resolution magic angle spinning (HRMAS) NMR [4]. We have also demonstrated the ability to observe and quantify chemical shift resonances from in vivo tendons using multi-echo UTE [5]. However, the relationship between these off-resonance signals and functional properties of tendon have not been clearly established. Shear Wave Elastography (SWE) Ultrasound (US) provides noninvasive and reliable quantification of Achilles tendon stiffness [6, 7]. The Achilles tendons (AT) of professional ballet dancers are adapted to withstand a high frequency of repeated mechanical loading compared to the general population. The purpose of this study was to examine the relationship between UTE-derived microstructural properties and SWE-derived tissue mechanical properties of healthy AT in professional ballet dancers.Literature Review
No literature exists for either SWE functional assessment or UTE MRI structural assessment in professional ballet dancers.Methods
This IRB-approved study recruited 15 professional ballet dancers. MRI was performed at 3T (Magnetom Prismafit; Siemens, Erlangen, Germany) using a 4-channel radio frequency coil, positioned around the dancer’s dominant ankle. Multi-echo UTE images were acquired using a prototype 3D stack of spirals sequence with 16 echoes ranging from a minimum echo time of 60µs to a maximum echo time of 30ms. Sequence parameters included 40ms repetition time, 30-degree excitation flip angle, 4 mm out of plane resolution and 0.625 mm in plane resolution. Regions of interest were selected in the UTE image to match the location of SWE tendon stiffness measurements. Multiexponential fitting of the UTE relaxation signals was performed:$$y(TE_n) = \beta_1e^{-R^*_{2,1}TE_n}+\beta_2e^{-R^*_{2,2}TE_n}+\beta_3e^{(R^*_{2,3}+j\omega_3)TE_n}e^{j\phi_3}$$ where $$$\beta_k, R^*_{2,k}$$$, $$$\omega_k$$$, and $$$\phi_k$$$ represent the amplitude fraction ($$$\sum\beta_k=1$$$), relaxation rate, frequency, and phase of the k-th component, respectively. Signals from on-resonance relaxation components, represented as β1 and β2, have been previously attributed to rapidly relaxing collagen-bound water and more slowly relaxing interstitial water, respectively [2]. SWE US measures were obtained using a 2D SWE instrument (Logiq s8 US machine; GE Healthcare, Little Chalfont, UK). Measurements were performed in two ankle positions: neutral-relaxed (NR) and under voluntary active maximum dorsiflexion (DF). Shear stiffness was recorded using wave velocity (m/s) with measurements made along the short axis (VS) and long axis (VL) relative to the AT (Fig. 1).
Results
One subject was excluded from the study based on UTE image quality. Average dancer age was 23.93±3.38 years. 8 (57%) females and 6 males (43%) with an average BMI of 19.97 (±1.68). All mid-substance signal decays exhibited substantial oscillations that were represented with minimal error using the three-component model (Fig. 2). The frequency ω3 of the off-resonance component was -389±375Hz from the water signal, consistent with our previous work in healthy non-athletic volunteers [5]. The somewhat large deviation in frequencies was driven by 6 subjects with frequencies larger than the mean value with the remaining 8 subjects showing frequencies consistent with the N-acetal resonance on proteoglycan side chains. No associations were observed when comparing SWE measurements from the NR ankle position to the multiexponential analysis. The long axis shear wave velocity (VL,DF) for the active maximum dorsiflexion SWE measurements were directly proportional to the β3 signal fraction (ρ=.58, p=.029, Fig. 3). No other associations were observed between UTE and SWE parameters.Discussion
The interpretation of off-resonance relaxation components in multi-echo UTE data has yet to be unambiguously determined. Previous work using HRMAS NMR in healthy and damaged tendon explant tissue have identified prominent signals assigned to chemical groups associated with collagen, proteoglycan, and other metabolites like lactate [4]. For a conservative representation approach, in light of the limited number of echoes and nonlinear echo spacing, we decided to represent the off-resonance signal as a single component. This oversimplification could potentially account for the increased variance of parameter estimates from this component. Interestingly, the relative amplitude of this off-resonance component correlates with tendon stiffness as assessed by SWE, suggesting its association with a matrix constituent of structural origin. Certainly, non-collagenous matrix molecules like proteoglycans have been shown to influence mechanical properties [8]. We note UTE and SWE observations from this group of professional dancers were obtained mid-season, so ongoing AT matrix remodeling is expected. Associated presence of chemical groups from cellular metabolites during this extracellular matrix turnover could also be influencing fit parameters. Further work that longitudinally evaluates this structure-function relationship over the course of the dance season could provide insight into microstructural and functional adaptations of the AT from intensive training. Ongoing work is also focused on characterizing these markers after acute injury to improve the quality of return to sport assessments.Acknowledgements
This research was funded in part by the Emory Orthopedics Research Pilot Grant program.References
- Filho G.H., et al. 2009 AJR, 192.3:W117-W124.
- Juras V. et al. 2012 Mag Res Med 68:1607-1613.
- Fullerton G.D. et al. 2007 J. Mag. Reson. Imag. 25:345-361.
- Swain A. et al. 2021 ISMRM Ann. Meeting #2981
- Anjum M. et al. 2021 MRM. 86:415-428.
- Baumer T.G. et al. 2017 J Biomech 53:201-204.
- Baumer T.G. et al. 2018 J Orthop Res 36;282-288.
- Robinson P.S. et al., J. Biomech. Eng., 2005, 127:181-185
Figures
Figure 1: Representative probe SWE US probe orientation
on the AT (depicted with black bar) showing measurements in the transverse and
longitudinal orientations.
Figure 2: Representative multi-echo UTE decay signal
from the AT mid-substance ROI with multiexponential fit.
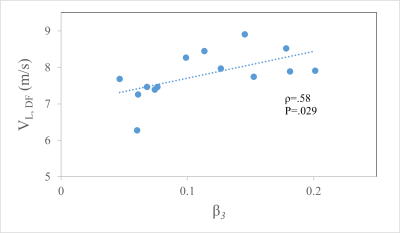
Figure 3: Positive association between off-resonance
signal amplitude β3 and SWE wave speed
in the longitudinal direction, reflecting an increase in stiffness with increasing
amplitude of matrix pool.
DOI: https://doi.org/10.58530/2023/1447