1434
A Multi-Nuclear MRI/MRS Study Exploring the Impact of a Novel Cardiac Mitotrope in the Treatment of Diabetic Cardiomyopathy1University of Oxford, Oxford, United Kingdom, 2Oxford University Hospitals NHS Foundations Trust, Oxford, United Kingdom, 3Imbria Pharmaceuticals, Boston, MA, United States, 4University College London, London, United Kingdom, 5Hamad Medical Corporation, Doha, Qatar, 6Slovak Academy of Sciences, Bratislava, Slovakia, 7Aarhus University, Aarhus, Denmark
Synopsis
Keywords: Heart, Diabetes, Multi-nuclear Spectroscopy
Patients with Type 2 Diabetes (T2D) are at a significantly increased risk of heart failure (HF) with the development of HF driven by energetic, metabolic, structural, and functional cardiac changes. In this study we have used a multi-nuclear MRI/MRS approach to demonstrate that the novel metabolic modulator, ninerafaxstat, can significantly improve myocardial energetics, cardiac steatosis and diastolic function in patients with T2D and obesity. In addition, using cardiac hyperpolarized [1-13C]pyruvate MRS, we have identified a potential signal for increased pyruvate dehydrogenase flux upon treatment with ninerafaxstat.Introduction
Patients with Type 2 Diabetes (T2D) are at a significantly increased risk of heart failure (HF) with the development of HF driven by energetic, metabolic, structural, and functional cardiac changes1. The T2D heart is metabolically inflexible and has an over-reliance on fatty acids for ATP generation, along with a reduced level of glucose oxidation caused by inhibition of mitochondrial pyruvate dehydrogenase (PDH) activity. This leads to a reduced efficiency of ATP generation and impaired myocardial energetics. T2D is also associated with myocardial steatosis, which contributes to lipotoxicity and LV diastolic dysfunction. In this study, we aimed to assess the effects of a novel cardiac mitotrope (ninerafaxstat) which is designed to shift cardiac substrate utilization away from fatty acid oxidation and towards glucose oxidation. Using a multi-nuclear MRI/MRS approach, we explored the effects of ninerafaxstat on cardiac energetics, substrate metabolism & diastolic function in participants with T2D and obesity.Methods
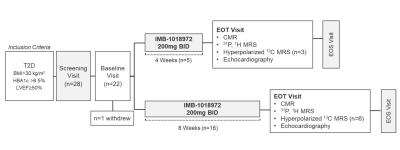
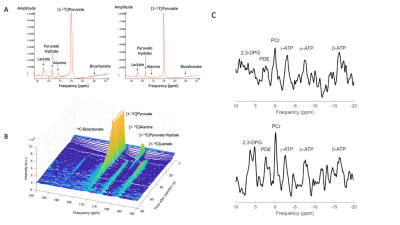
As shown in Figure 1, 21 participants with T2D and obesity (median HbA1c 7.0% [interquartile range (IQR) 6.6, 7.8], body mass 97 kg [90,102]) received 200 mg ninerafaxstat twice daily for 4 (n=5) or 8 weeks (n=16). Myocardial energetics (the primary endpoint), metabolism and function were assessed pre- & post-treatment using MRI, 31P- and 1H MRS in all patients, with assessment of PDH flux using hyperpolarized [1-13C]pyruvate MRS acquired in a subset (n=9, Figure 2). Data were acquired at 3T (MAGNETOM Trio, Siemens Healthineers, Erlangen, Germany). Short axis CINE images covering the whole left ventricle (LV) were acquired using BSSFP imaging. Scan parameters were typically: TR/TE=40.5/1.14ms, flip angle=55°, FOV=380x380mm, voxel size=2.0x2.0x8.0mm. 31P-MRS was performed at rest and during dobutamine stress with participants positioned prone over the centre of a 3-element dual-tuned 1H/31P surface coil (Siemens Medical, Erlangen, Germany). A non-gated 3D acquisition-weighted ultra-short echo time CSI sequence was used with saturation bands placed over liver and skeletal muscle2. The reported PCr/ATP was averaged over two basal septal voxels and corrected for blood signal contamination. 1H-MRS assessments were performed as previously described3 with end-expiration, ECG-triggered spectra obtained from the mid inter-ventricular septum with and without water suppression to measure myocardial triglyceride (MTG) content. MTG content was calculated as a percentage (signal amplitude of lipid/signal amplitude of water). Assessment of PDH flux was undertaken as previously described4 following injection of 0.4 mL/kg of hyperpolarized [1-13C]pyruvate solution at a rate of 5 mL per second. Slice selective 13C spectra were acquired every second for 4 minutes following injection. Integrated metabolite-to-pyruvate ratios, known to linearly correlate with first-order chemical kinetic rate constants, were calculated from 60 seconds of data taken after the initial appearance of the pyruvate resonance in the spectrum.Results
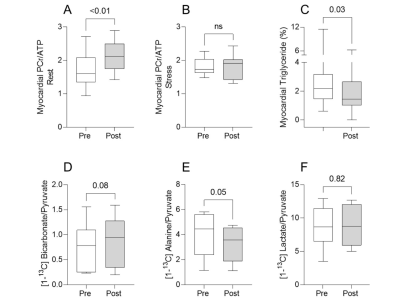
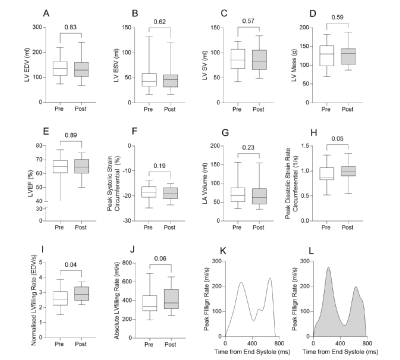
In keeping with previous results, patients with T2D presented with reduced PCr/ATP (1.6 [1.4, 2.1]), myocardial steatosis (myocardial triglycerides 2.2% [1.5, 3.2]), left ventricular (LV) hypertrophy (LV mass 130g [98, 152]), and diastolic dysfunction (peak diastolic strain rate 0.86 s-1 [0.82, 1.06]). Figures 3 & 4 show that ninerafaxstat significantly improved myocardial energetics (PCr/ATP median by 32%, p<0.01), reduced myocardial triglyceride content (by 34%, p=0.03) and improved diastolic function (peak diastolic strain rate by 10%, peak LV filling rate by 11%, both p<0.05). The bicarbonate:pyruvate ratio, indicative of PDH flux, was increased in 7/9 subjects (mean 45%, p=0.08). Left ventricular volumes and mass, heart rate and blood pressure remained unchanged.Discussion
In this open-label, proof-of-mechanism trial, we evaluated the impact of ninerafaxstat, a novel metabolic modulator designed to improve the efficiency of ATP generation, on myocardial energetics, metabolism, and function in participants with T2D and obesity. Using 31P MRS, we observed that 4-8 weeks treatment with ninerafaxstat was sufficient to improve myocardial energetics (PCr/ATP). Using cardiac hyperpolarized [1-13C]pyruvate MRS for the first time in a clinical trial of an novel therapeutic agent, we identified a potential signal for increased PDH flux on treatment with ninerafaxstat. Furthermore, using 1H spectroscopy, we showed that ninerafaxstat reduced the increased myocardial triglyceride content that is a hallmark feature of both T2D and obesity. Finally, using feature-tracking CMR, we demonstrated that these alterations in myocardial metabolism and energetics translate into improved diastolic function.Conclusion
Treatment with the novel mitotrope, ninerafaxstat, significantly improved myocardial energetics, measures of steatosis and diastolic filling in patients with T2D and obesity.Acknowledgements
DT acknowledges salary support from a British Heart Foundation Senior Fellowship (FS/19/18/34252). MH acknowledges salary support from Imbria pharmaceuticals. LV is supported by a Sir Henry Dale Fellowship, jointly funded by the Royal Society and the Wellcome Trust (221805/Z/20/Z), and also acknowledges support of the Slovak Grant Agencies VEGA (2/0003/20) and APVV (#19-0032). JM was supported by a Novo Nordisk Postdoctoral Fellowship run in conjunction with the University of Oxford. OR acknowledges salary support from a British Heart Foundation Senior Fellowship (FS/SCRF/22/32014).
We further acknowledge support from the British Heart Foundation Oxford Centre of Research Excellence and from the NIHR Oxford Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
References
(1) Seferovic PM, Petrie MC, Filippatos GS, Anker SD, Rosano G, Bauersachs J, Paulus WJ, Komajda M, Cosentino F, de Boer RA, Farmakis D, Doehner W, Lambrinou E, Lopatin Y, Piepoli MF, Theodorakis MJ, Wiggers H, Lekakis J, Mebazaa A, Mamas MA, Tschope C, Hoes AW, Seferovic JP, Logue J, McDonagh T, Riley JP, Milinkovic I, Polovina M, van Veldhuisen DJ, Lainscak M, Maggioni AP, Ruschitzka F and McMurray JJV. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20:853-872.
(2) Tyler DJ, Emmanuel Y, Cochlin LE, Hudsmith LE, Holloway CJ, Neubauer S, Clarke K and Robson MD. Reproducibility of 31P cardiac magnetic resonance spectroscopy at 3 T. NMR Biomed. 2009;22:405-13.
(3) Rial B, Robson MD, Neubauer S and Schneider JE. Rapid quantification of myocardial lipid content in humans using single breath-hold 1H MRS at 3 Tesla. Magnetic Resonance in Medicine. 66:619-624.
(4) Rider OJ, Apps A, Miller J, Lau JYC, Lewis AJM, Peterzan MA, Dodd MS, Lau AZ, Trumper C, Gallagher FA, Grist JT, Brindle KM, Neubauer S and Tyler DJ. Non-invasive In Vivo Assessment of Cardiac Metabolism in the Healthy and Diabetic Human Heart Using Hyperpolarized 13C MRI. Circ Res. 2020;126:725-736.
Figures