1433
Free-breathing simultaneous myocardial T2 and T1ρ mapping for non-contrast assessment of uremic cardiomyopathy
1School of Biomedical Engineering, ShanghaiTech University, Shanghai, China, 2Shanghai Clinical Research and Trial Center, Shanghai, China, 3Department of Cardiovascular Medicine, Ruijin Hospital Lu Wan Branch, Shanghai Jiao Tong University School of Medicine, Shanghai, China, 4UIH America, Inc., Houston, TX, United States
Synopsis
Keywords: Myocardium, Cardiomyopathy
Uremic cardiomyopathy is the adverse cardiac remodeling that commonly occurs in patients with chronic kidney disease. Previous studies have indicated increased intramyocardial fluid and myocardial fibrosis in uremic patients, which makes native myocardial T2 and T1ρ mapping ideal imaging biomarkers to characterize these changes. Therefore, we propose a free-breathing simultaneous T2 and T1ρ mapping technique to provide co-registered T2 and T1ρ maps. The proposed technique was firstly evaluated in phantoms and ten healthy subjects, which achieved similar performance to the conventional separate T2 and T1ρ mapping methods. The preliminary validation in four hemodialysis patients showed promising results.
Introduction
Cardiovascular disease (CVD) is one of the major complications of chronic kidney disease (CKD), and uremic cardiomyopathy (UC) is the leading cause of CVD deaths in CKD patients1,2. Previous studies have indicated increased intramyocardial fluid and myocardial fibrosis in uremic patients3, which may play a role in the development of UC. Native myocardial T2 and T1ρ mapping can respectively be used to detect edema4 and diffuse myocardial fibrosis5,6, and can be used to evaluate the myocardial tissue changes in CKD. The clinically available mapping techniques quantified myocardial T2 and T1ρ in two separate breath-hold scans, which may result in mis-registration between the parametric maps due to different breath-hold positions. This study sought to propose a joint T2 and T1ρ mapping technique, which has to be performed under free-breathing due to prolonged acquisition time and prospective and retrospective motion correction (MoCo) were adopted. The proposed technique achieved similar performance to the conventional separate T2 and T1ρ mapping methods in phantoms and healthy subjects, and showed promising results in four hemodialysis patients.Methods
The sequence diagram of the proposed technique is shown in Fig. 1, which consists of seven bSSFP based single-shot acquisitions between which there are three empty cardiac cycles for signal recovery7, resulting in a total of 25 heartbeats. T2 preparation (T2-prep) with three different echo times (TE) and T1ρ preparation (T1ρ-prep) with four different spin-lock durations (TSL) are interleaved performed before the acquisition, where the module with short TE or TSL is performed first to reduce the influence of incomplete recovery of longitudinal relaxation on parameter quantification. The T1ρ-prep module is designed with adiabatic excitation pulses to be robust to field inhomogeneities at 3T8. To enable free-breathing acquisition, the diaphragm navigator (dNav) is performed before the preparation pulse to adjust the imaging slice in real time and compensate for the through-plane respiratory motion9. The in-plane motion between the multiple contrasts is corrected with a PCA-based groupwise registration approach10. T2 and T1ρ are fitted separately with the two-parameter exponential decay model7,8.Experiments
All imaging experiments were conducted on a 3T United Imaging scanner. The phantoms made of different concentrations of agarose were imaged with the proposed sequence, in comparison with the reference sequences: multi-echo spin-echo (MESE) for T2 and T1ρ prep gradient echo (T1ρ-GRE) for T1ρ8. The proposed technique was performed with simulated heart rates from 40 to 120 bpm with a step of 20 bpm to investigate how heart rates affect its measurements. The conventional T2-prep bSSFP (T2-bSSFP)11 and T1ρ bSSFP (T1ρ-bSSFP)8 were also performed at 80 bpm, where the TE, TSL values and the number of the empty cardiac cycles were set the same to the joint mapping technique. Other imaging parameters for the mapping sequences were: FOV=320 × 280mm2, in-plane resolution=2.02 × 1.82mm2, thickness = 8mm, TR/TE/flip angle=3.01ms/1.5ms/35°, bandwidth=1200 Hz/px, GRAPPA acceleration factor=2.After institutional review board approval and obtaining written informed consent, ten healthy subjects (10 males; 24 years) and four patients (3 males, 1 female; 44 years) were imaged using the proposed technique at three short-axis slices with same imaging parameters provided in the phantom study. To evaluate the effects of motion correction, the proposed technique with dNav and in-plane MoCo (dNav-MoCo) were compared with the dNav acquisition without in-plane MoCo (dNav) and the free-breathing acquisition without dNav but with in-plane MoCo (FB-MoCo). The breath-hold T2-bSSFP and T1ρ-bSSFP were performed in the healthy subjects as reference. The mean T2 and T1ρ calculated in the mid short-axis slice were compared between different mapping methods using One-Way ANOVA. For segment-wise analysis, the American Heart Association’s (AHA) 16-segment model was adopted12.
Results
In phantoms, the joint mapping method agreed well with the breath-hold separate mapping methods acquired at 80bpm, while compared with the reference methods, it tends to overestimate T2 and T1ρ and the overestimation increases for long T2 and T1ρ values at low heart rates (Fig. 2). Example in vivo mapping results are shown in Fig. 3, where the dNav-MoCo achieved similar mapping quality to the breath-hold techniques and outperformed dNav and FB-MoCo, indicating the effectiveness of both through-plane and in-plane MoCo. This finding is also echoed in Fig. 4, where dNav-MoCo largely reduced the measurement variance. Compared with separate mapping methods, the joint mapping method resulted in higher T2 and T1ρ values, which may be caused by incomplete signal recovery in the interleaved T2 and T1ρ-prep acquisition. The Bullseye plots and example patients results are shown in Fig. 5, higher T2 and T1ρ values are observed for the uremic patients than the healthy subjects.Discussion
We have developed a free-breathing simultaneous T2 and T1ρ mapping technique. Its accuracy and the motion correction effectiveness have been validated in phantoms and healthy subjects. The clinical feasibility has been preliminarily demonstrated in uremic patients, where higher T2 and T1ρ values in the patients than the healthy subjects were observed. Future work will recruit more uremic patients to investigate the clinical value of the proposed method.Acknowledgements
No acknowledgement found.References
1 Briet, M., Boutouyrie, P., Laurent, S. & London, G. M. Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int 82, 388-400 (2012).
2 Edwards, N. C. et al. Defining the natural history of uremic cardiomyopathy in chronic kidney disease: the role of cardiovascular magnetic resonance. JACC Cardiovasc Imaging 7, 703-714 (2014).
3 Semple, D., Smith, K., Bhandari, S. & Seymour, A. M. Uremic cardiomyopathy and insulin resistance: a critical role for akt? J Am Soc Nephrol 22, 207-215 (2011).
4 Gottbrecht, M., Kramer, C. M. & Salerno, M. Native T1 and Extracellular Volume Measurements by Cardiac MRI in Healthy Adults: A Meta-Analysis. Radiology 290, 317-326 (2019).
5 von Knobelsdorff-Brenkenhoff, F. et al. Myocardial T1 and T2 mapping at 3 T: reference values, influencing factors and implications. J Cardiovasc Magn Reson 15, 53 (2013).
6 Puntmann, V. O., Peker, E., Chandrashekhar, Y. & Nagel, E. T1 Mapping in Characterizing Myocardial Disease: A Comprehensive Review. Circ Res 119, 277-299 (2016).
7 Akcakaya, M. et al. Improved quantitative myocardial T2 mapping: Impact of the fitting model. Magn Reson Med 74, 93-105 (2015).
8 Qi, H. et al. Accelerated 3D free-breathing high-resolution myocardial T1rho mapping at 3 Tesla. Magn Reson Med 88, 2520-2531 (2022).
9 Basha, T. A. et al. Free-breathing cardiac MR stress perfusion with real-time slice tracking. Magn Reson Med 72, 689-698 (2014).
10 Huizinga, W. et al. PCA-based groupwise image registration for quantitative MRI. Med Image Anal 29, 65-78 (2016).
11 Huang, T. Y., Liu, Y. J., Stemmer, A. & Poncelet, B. P. T2 measurement of the human myocardium using a T2-prepared transient-state TrueFISP sequence. Magn Reson Med 57, 960-966 (2007).
12 Baessler, B. et al. A systematic evaluation of three different cardiac T2-mapping sequences at 1.5 and 3T in healthy volunteers. Eur J Radiol 84, 2161-2170 (2015).
Figures
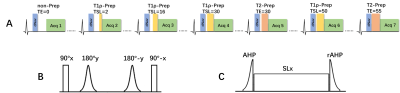
Fig. 1 A: Pulse sequence for the proposed free-breathing T2 and T1ρ mapping technique with diaphragmatic navigator (dNav) to adjust the imaging slice in real time. The parameters of preparation modules are: T2-prep TEs = {0, 30, 55} ms and T1ρ-prep spin-lock times (TSL) = {2, 16, 30, 50} ms at SL frequency = 350 Hz. B: The T2-prep module. C: The T1ρ-prep module, consisting of an adiabatic half-passage (AHP) tip-down pulse, a continuous-wave spin-lock pulse, and a reverse AHP tip-up pulse (rAHP).
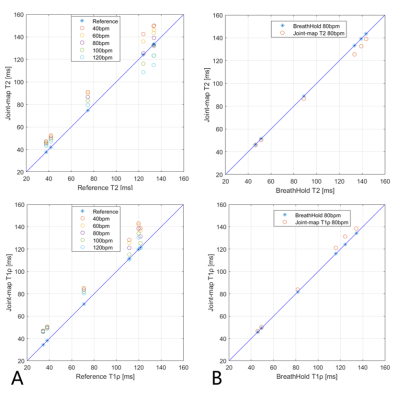
Fig. 2 A: Phantom T2 and T1ρ values measured with the joint mapping method with heart rates from 40 to 120 bpm, compared with reference methods. B: Comparison of phantom T2 and T1ρ measured with joint mapping method with the separate mapping methods, T2-bSSFP and T1ρ-bSSFP at 80bpm.
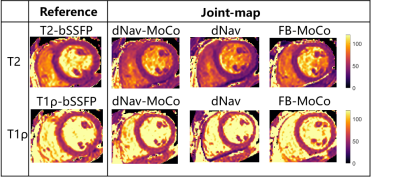
Fig. 3 Example T2 and T1ρ maps from a healthy subject with breath-hold T2-bSSFP and T1ρ-bSSFP and Joint mapping methods: dNav-MoCo, dNav, FB-MoCo.
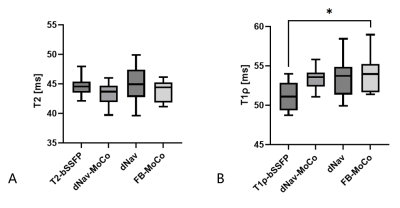
Fig. 4 Comparison of mean T1ρ (A) and T2 (B) values in the mid short-axis slice estimated withbreath-hold T2-bSSFP and T1ρ-bSSFP, and joint mapping methods: dNav-MoCo, dNav, FB-MoCo, where * indicates statically significant with p<0.05.
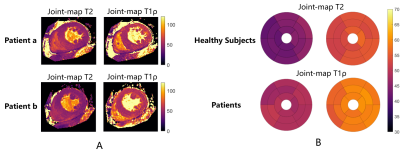
Fig. 5 A, Example T2 and T1ρ maps from two uremic patients. B, Bullseye plots comparing mean T2 and T1ρ values averaged across healthy volunteers and those of uremic patients with the proposed joint mapping technique.