1431
Myofiber strain estimation using cDTI, DENSE, and feature tracking.1University of Lyon, UJM-Saint-Etienne, INSA, CNRS UMR 5520, INSERM U1206, CREATIS, F-42023, Saint-Etienne, France, 2Department of Radiology, University Hospital Saint-Etienne, Saint-Etienne, France, 3Department of Mechanical and Aerospace Engineering, University of Central Florida, Orlando, FL, United States
Synopsis
Keywords: Myocardium, Cardiovascular, Cardiac Function, cDTI, DENSE, Feature Tracking, cardiac microstructure
Myofiber strain (MS) is a promising biomarker of cardiac function, but it requires the combination of cDTI and of a 3D cardiac displacement field. Displacement fields can be measured using DENSE imaging, but with low spatial resolution and limited spatial coverage. Feature tracking (FT) allows the estimation of the displacement directly from cine imaging. In this study, myofiber strains estimated using DENSE and FT were compared on thirty healthy volunteers. The magnitude of myofiber strain calculated with DENSE was higher than with FT (MSDENSE=-0.15[-0.16;-0.14] vs MSFT=-0.11[-0.14;-0.06], p<0.001) but no correlation was found between MSDENSE and MSFT (r=0.14 p=0.47).Background
The cardiac cells (cardiomyocytes) branch and connect along preferential directions to form so-called “myofibers”1. In the left ventricle (LV), myofibers change orientation in a helical fashion from epicardium to endocardium2. This unique architecture transforms the uniaxial cellular shortening into the complex 3D deformation of the LV.Cardiac motion can be evaluated in MR using a phase contrast approach such as cine DENSE imaging3,4, which provides quantitative spatiotemporally resolved maps of myocardial tissue displacement. However DENSE requires a long acquisition time, which usually limits spatial resolution and heart coverage. Alternatively, cardiac motion can be estimated using a feature tracking (FT) algorithm applied to traditional cine imaging5. FT benefits from the good spatial coverage and resolution of cine imaging but relies on several assumptions to estimate cardiac displacement.
Despite being intrinsically linked to the cardiomyocytes’ deformation and organization, cardiac function is often measured in an arbitrary cylindrical coordinate system along the circumferential, longitudinal, and radial directions without considering the cardiomyocytes’ orientation. Measuring cardiac function considering the cardiac microstructure remains difficult as it requires measuring the myofiber organization6. The recent development of motion-compensated diffusion encoding designs has enabled Cardiac Diffusion Tensor imaging (cDTI)7. cDTI has the unique capability of estimating myofiber orientation in vivo.
By retrospectively combining myofiber orientations obtained from cDTI and 3D cardiac displacement field from DENSE, we previously demonstrated the possibility of measuring myofiber strain8,9. Myofiber strain represents the cardiac deformation along the direction of cardiomyocytes aggregates and thus directly represents cardiomyocytes’ shortening.
We propose to evaluate myofiber strain using cDTI and FT as FT may improve the feasibility of this approach in the clinic by only requiring an extra cDTI acquisition to estimate myofiber strains. In this work, traditional cylindrical strains as well as myofiber strain computed from DENSE and FT were measured and compared in thirty (N=30) healthy volunteers.
Methods
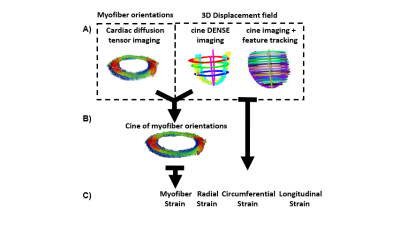
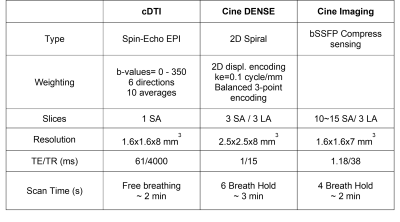
Acquisition protocol - Thirty healthy volunteers (N=30) were imaged at 3T (Prisma, Siemens) following IRB approval and informed consent. The sequence parameters for cDTI, DENSE, and the bSSFP cine10 are given in Table 1.Strain estimation - As shown in Figure 1, myofiber strain is computed using cDTI data and a displacement field. In this work, displacement fields were obtained either directly from DENSE imaging or by applying an FT algorithm (CVI42, Circle Cardiovascular Imaging) on bSSFP cine acquisitions. For both FT and DENSE, 2D displacement fields were derived for each SA and LA slice from which baseline global radial (GRS), circumferential (GCS), and longitudinal strain (GLS) were calculated (Figure 1-C) . The 2D displacement fields were then combined to obtain an LV 3D displacement field. As shown in Figure 1-B, the 3D displacement fields for DENSE and FT were applied to the myofiber orientations measured with cDTI to obtain a cine of myofiber orientation through the cardiac cycle and then estimate myofiber strain (MS). The myofiber reconstruction pipeline is available at https://github.com/KMoulin/Eff.
Analysis - Baseline GRS, GCS, GLS, and MS calculated from DENSE and FT were compared across volunteers and reported as median [interquartile range]. Differences between median strains were evaluated using a Wilcoxon rank test (p<0.05).
Results
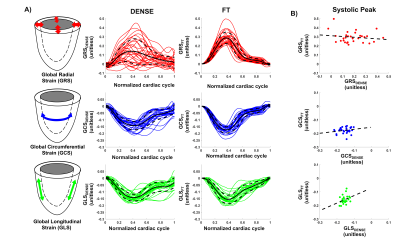
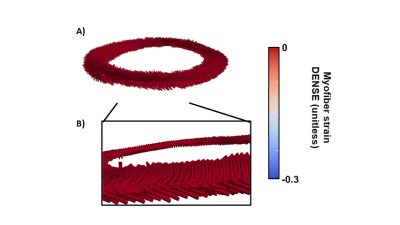
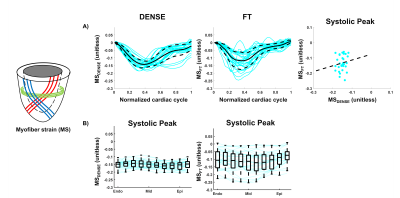
Baseline Strains - GRS, GCS, and GLS for DENSE and FT across volunteers are shown in Figure 2-A-B. At peak systole, radial and circumferential strains estimated with DENSE had a smaller magnitude than when estimated using FT (GRSDENSE = 0.14 [0.05;0.23] vs GRSFT = 0.25 [0.21;0.30], p<0.01, GCSDENSE = -0.14 [-0.18;-0.12] vs GCSFT = -0.16 [-0.18;-0.15], p= 0.34). Longitudinal strains were equivalent for DENSE and FT (GLSDENSE = -0.15 [-0.16;-0.13] vs GLSFT = -0.15 [-0.17;-0.13], p=0.77). As shown in Figure 2-C, no significant correlations of strain magnitudes at peak systole were found between DENSE and FT (r=0.21 p=0.26 for GRS, r=-0.16 p=0.4 for GCS, r=0.32 p=0.11 for GLS).Myofiber Strains - An animated example of a cine of myofiber orientation is reported in Figure 3-A. The resulting myofiber shortenings are represented in Figure 3-B. DENSE and FT Myofiber strains are displayed in Figure 4. The distribution of myofiber strains was wider for FT. At peak systole, the magnitude of myofiber strain calculated with DENSE was higher in absolute value than when computed using FT (MSDENSE = -0.15 [-0.16;-0.14] vs MSFT = -0.11 [-0.14;-0.06], p<0.001). No correlation was found between DENSE and FT myofiber strain (r=0.14 p=0.47 for MS). Transmural peak systolic strain distribution for MSDENSE and MSFT are shown in Figure 4-B. For both DENSE and FT, peak strains were transmurally uniform.
Discussion and Conclusion
In this study, in vivo myofiber strains were computed in healthy volunteers using cDTI, DENSE, and FT. Myofiber strain is a promising biomarker to study cardiac function as it represents directly cardiomyocyte shortening. Even though larger variability was obtained for FT myofiber strain, uniform transmural distribution was still observed at peak systole similar to DENSE myofiber strain. With improvement on FT accuracy, MSFT may be sufficient to identify a deviation from uniform transmural MS to detect the onset and progression of cardiac dysfunction. FT myofiber strain represents an interesting alternative to DENSE as it does not require additional image acquisitions to measure the ventricles’ displacement field.Acknowledgements
The authors want to thank Mihaela Chirvasa from Circle Cardiovascular Imaging for her valuable technical support.
This material is based upon work partially supported by the National Science Foundation under Grant No. 2205043 to LEP.
References
1. Axel L, Wedeen VJ, Ennis DB. Probing dynamic myocardial microstructure with cardiac magnetic resonance diffusion tensor imaging. Journal of cardiovascular magnetic resonance. 2014 Dec;16(1):1-7.
2. Streeter Jr DD, Spotnitz HM, Patel DP, Ross Jr J, Sonnenblick EH. Fiber orientation in the canine left ventricle during diastole and systole. Circulation research. 1969 Mar;24(3):339-47.
3. Aletras AH, Ding S, Balaban RS, Wen H. DENSE: displacement encoding with stimulated echoes in cardiac functional MRI. Journal of magnetic resonance (San Diego, Calif.: 1997). 1999 Mar;137(1):247.
4. Zhong X, Spottiswoode BS, Meyer CH, Kramer CM, Epstein FH. Imaging three‐dimensional myocardial mechanics using navigator-gated volumetric spiral cine DENSE MRI. Magnetic resonance in medicine. 2010 Oct;64(4):1089-97.
5. Yang W, Li H, He J, Yin G, An J, Forman C, Schmidt M, Zhao S, Lu M. Left Ventricular Strain Measurements Derived from MR Feature Tracking: A Head-to-Head Comparison of a Higher Temporal Resolution Method With a Conventional Method. Journal of Magnetic Resonance Imaging. 2022 Jan 10.
6. Tseng WY, Reese TG, Weisskoff RM, Brady TJ, Wedeen VJ. Myocardial fiber shortening in humans: initial results of MR imaging. Radiology. 2000 Jul;216(1):128-39.
7. Stoeck CT, Von Deuster C, Genet M, Atkinson D, Kozerke S. Second-order motion-compensated spin echo diffusion tensor imaging of the human heart. Magnetic resonance in medicine. 2016 Apr;75(4):1669-76.
8. Verzhbinsky IA, Perotti LE, Moulin K, Cork TE, Loecher M, Ennis DB. Estimating aggregate cardiomyocyte strain using In Vivo diffusion and displacement encoded MRI. IEEE transactions on medical imaging. 2019 Aug 8;39(3):656-67.
9. Moulin K, Croisille P, Viallon M, Verzhbinsky IA, Perotti LE, Ennis DB. Myofiber strain in healthy humans using DENSE and cDTI. Magnetic Resonance in Medicine. 2021 Jul;86(1):277-92.
10. Lin AC, Strugnell W, Riley R, Schmitt B, Zenge M, Schmidt M, Morris NR, Hamilton‐Craig C. Higher resolution cine imaging with compressed sensing for accelerated clinical left ventricular evaluation. Journal of Magnetic Resonance Imaging. 2017 Jun;45(6):1693-9.
Figures