1427
Regional quantification of cardiac metabolism with hyperpolarized [1-13C]-pyruvate MRI1Radiology and Biomedical Imaging, University of California - San Francisco, San Francisco, CA, United States, 2HeartVista, Palo Alto, CA, United States, 3University of Colorado Anschutz, Aurora, CO, United States, 4University of Pennsylvania, Philadelphia, PA, United States, 5University of Washington, Seattle, WA, United States
Synopsis
Keywords: Myocardium, Metabolism
Hyperpolarized (HP) 13C-pyruvate MRI is a promising new tool for non-invasive quantification of myocardial glycolytic and Krebs cycle metabolism. In this study we evaluated whole-heart imaging and metabolism quantification methods in 7 healthy volunteers under a fasted and fed state. We observed that the 13C-pyruvate-to-bicarbonate conversion rate, kPB, a measure of PDH flux, had the highest, statistically significant correlation with blood glucose levels, with smaller changes in the 13C-lactate/pyruvate ratio and 13C-pyruvate-to-lactate conversion rate, kPL. 13C-pyruvate and 13C-lactate were detected simultaneously in the RV blood pool, immediately after intravenous injection, reflecting LDH activity in blood.
Introduction
Cardiac contraction imposes very high energy demands on the heart. Changes in substrate and oxygen availability, cardiac work, and sarcomeric protein gene mutations causing cardiomyopathies1 lead to changes in cardiac energy metabolism, which cause secondary changes in gene expression and structural remodeling, that predispose to heart failure and arrhythmias. Hence, in vivo imaging of cardiac metabolism in humans could be helpful for presymptomatic detection of cardiomyopathy, assessing response to therapies and prognosis. With hyperpolarized [1-13C]-pyruvate MRI, the relative contribution of glucose oxidation and fatty acid oxidation to energy production in the heart can be assessed by [1-13C]-pyruvate conversion to [1-13C]-bicarbonate, catalyzed by pyruvate dehydrogenase (PDH), and [1-13C]-lactate, catalyzed by lactate dehydrogenase (LDH)2-10.Methods
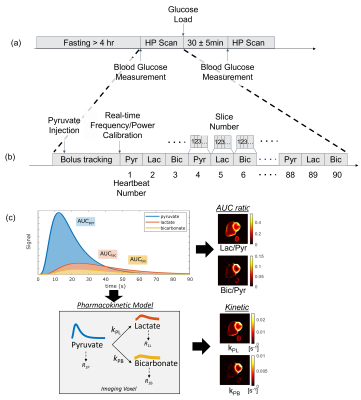
The key components of the study are illustrated in Figure 1. Seven healthy volunteers with no known cardiovascular disease history or contraindications to MRI were recruited with UCSF Institutional Review Board approval. The study design included a modest oral glucose challenge between 2 HP scans, where an oral glucose load (drinking a bottle of Gatorade containing 30g total carbohydrates) was administered after the first scan.The hyperpolarized MRI scan was performed using a multi-slice, multi-metabolite imaging sequence in an autonomous scanning protocol11 which included: (1) automatic triggering based on bolus arrival in the right ventricle (RV) cavity (2) a real-time 13C frequency update and (3) measurement and adjustment of the 13C transmit power. Subjects were placed in a Helmholz “clamshell” transmit coil and an 8-channel “paddle” receive array12. Scans used: 6 mm x 6 mm in-plane resolution for pyruvate, 12 mm x 12 mm in-plane resolution for bicarbonate and lactate13, 21 mm slice thickness, 5 slices, a temporal resolution of 3 heartbeats (~3.6s), 20o excitation flip angle for pyruvate, and 30o excitation flip angle for lactate and bicarbonate.
Quantification of the metabolic images was performed using from area-under-the-curve (AUC) images, and a pharmacokinetic model. Kinetic rates for conversion from pyruvate to lactate (kPL) and pyruvate to bicarbonate (kPB) were fit to a uni-directional, three-site pharmacokinetic model with one physical compartment14 using an “inputless” approach15.
The coil combination, phasing methods, and pharmacokinetic model fitting are available in the hyperpolarized-mri-toolbox16.
Results
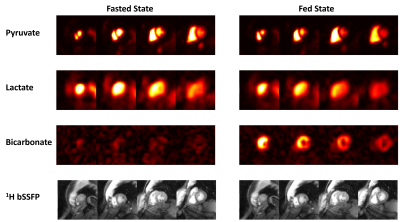
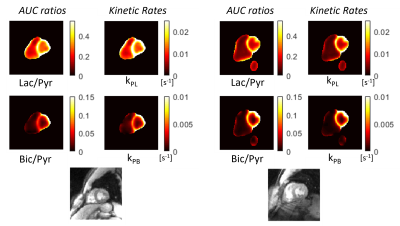
Figure 2 shows representative hyperpolarized 13C AUC images for both the fasting and fed states in a healthy volunteer. This distribution between the cavities and myocardium was consistent across studies.The 13C-lactate/13C-pyruvate and 13C-bicarbonate/13C-pyruvate AUC ratio maps in Fig. 4 show that this approach leads to clearer depiction in myocardium compared to the metabolite AUC maps alone. Kinetic rate maps from pharmacokinetic modeling in Fig. 4 show that, similarly to the AUC ratio maps, this leads to a clear depiction of the myocardium and relatively homogeneous maps.
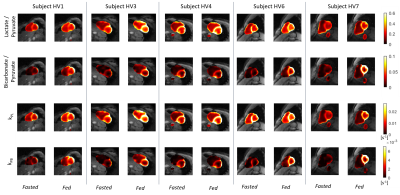
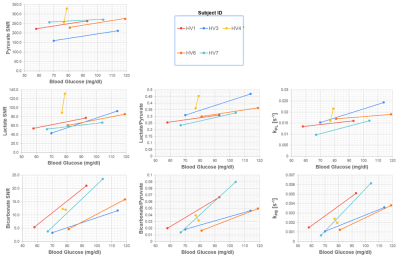
Fig. 5 shows the AUC ratios and kinetic rates in all healthy volunteers before and after oral glucose load. In all cases except one, there was uniform increase in the 13C-bicarbonate/13C-pyruvate and kPB maps in LV myocardium.
Figure 6 shows correlations between blood glucose levels and 13C measurements in LV myocardium between fed and fasted states. There were statistically significant changes in 13C-pyruvate SNR, 13C-lactate SNR, 13C-bicarbonate SNR, 13C-lactate/pyruvate, kPL and kPB between the fasted to the fed state (paired T-test, p < 0.05). The strongest correlations with blood glucose levels were observed for 13C-bicarbonate SNR (r = 0.50), 13C-bicarbonate/13C-pyruvate ratio (r = 0.54) and kPB (r = 0.56). The correlation was only statistically significant (p < 0.05) for kPB.
Discussion
Both AUC metabolite ratios and kinetic rates can correct for RF receive coil profiles as well as variations in 13C-pyruvate polarization, concentration, and delivery17. The pharmacokinetic modeling has theoretical advantages of more robust measurements than AUC ratios with different experimental parameters or conditions such as timings and flip angles15. While the AUC ratio and kinetic rate quantifications were relatively similar, we did observe that the kinetic rate, kPB, had the highest correlation with blood glucose levels and was the only correlation that was statistically significant.We observed clear evidence of 13C-lactate in the blood pool at the same time as the 13C-pyruvate bolus, with both metabolites first simultaneously in the RV. This can be explained by conversion of 13C-pyruvate to 13C-lactate via LDH in circulating red blood cells that is occurring as soon as the injection begins18,19. This potentially has an impact on all hyperpolarized 13C-pyruvate studies, as it implies that the 13C-lactate observed can be coming from blood pool metabolic conversion instead of the tissue of interest. This circulating 13C-lactate could also be a source of contrast as it can be used as a fuel source by the myocardium20–22.
Conclusion
We demonstrated regional quantification of LDH and PDH mediated metabolic conversion where both ratiometric and kinetic modeling-based quantifications showed high quality maps with metabolism primarily localized to LV myocardium. Almost all 13C measurements in LV myocardium (pyruvate SNR, lactate SNR, bicarbonate SNR, lactate/pyruvate ratio, kPL, and kPB) had significant increases following an oral glucose load, with the largest changes in measurements representing PDH activity. Blood glucose levels were valuable to account for a non-responder to the oral glucose challenge. kPB, a measure of PDH activity, had the largest correlation with blood glucose levels, and was the only measurement with a statistically significant correlation.Acknowledgements
We would like to acknowledge assistance with hyperpolarized experiments from Kimberly Okamoto, Mary Frost, Heather Daniel, James Slater, Andrew Riselli, Evelyn Escobar, and Romelyn Delos Santos.
This work was supported by funding from a UCSF Resource Allocation Program Team Science award, Myokardia Inc. Myoseeds program and NIH grants R33HL161816, P41EB013598, and U24CA253377. ND received post-doctoral training funding from the American Heart Association (grant number 20POST35200152).
References
1. Vakrou S, Abraham MR. Hypertrophic cardiomyopathy: a heart in need of an energy bar? Front Physiol. 2014;5:309. PMCID: PMC4137386
2. Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PEZ, Harzstark AL, Ferrone M, van Criekinge M, Chang JW, Bok R, Park I, Reed G, Carvajal L, Small EJ, Munster P, Weinberg VK, Ardenkjaer-Larsen JH, Chen AP, Hurd RE, Odegardstuen LI, Robb FJ, Tropp J, Murray JA. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized [1-13C]Pyruvate. Sci Transl Med. 2013 Aug;5(198):198ra108. PMID: 23946197
3. Cunningham CH, Lau JYC, Chen AP, Geraghty BJ, Perks WJ, Roifman I, Wright GA, Connelly KA. Hyperpolarized 13C Metabolic MRI of the Human HeartNovelty and Significance: Initial Experience. Circ Res. 2016 Nov 11;119(11):1177–1182. PMID: 27635086
4. Reed GD, Ma J, Park JM, Schulte RF, Harrison CE, Chen AP, Pena S, Baxter J, Derner K, Tai M, Raza J, Liticker J, Hall RG, Sherry AD, Zaha VG, Malloy CR. Characterization and compensation of inhomogeneity artifact in spiral hyperpolarized 13C imaging of the human heart. Magnetic Resonance in Medicine [Internet]. [cited 2021 Feb 8];n/a(n/a). Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/mrm.28691
5. Ma J, Chen J, Reed GD, Hackett EP, Harrison CE, Ratnakar J, Schulte RF, Zaha VG, Malloy CR, Park JM. Cardiac measurement of hyperpolarized 13C metabolites using metabolite-selective multi-echo spiral imaging. Magnetic Resonance in Medicine [Internet]. [cited 2021 Apr 8];n/a(n/a). Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/mrm.28796
6. Ma J, Malloy CR, Pena S, Harrison CE, Ratnakar J, Zaha VG, Park JM. Dual-phase imaging of cardiac metabolism using hyperpolarized pyruvate. Magnetic Resonance in Medicine [Internet]. [cited 2021 Oct 15];n/a(n/a). Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/mrm.29042
7. Joergensen SH, Hansen ESS, Bøgh N, Bertelsen LB, Staehr PB, Schulte RF, Malloy C, Wiggers H, Laustsen C. Detection of increased pyruvate dehydrogenase flux in the human heart during adenosine stress test using hyperpolarized [1-13C]pyruvate cardiovascular magnetic resonance imaging. Journal of Cardiovascular Magnetic Resonance. 2022 Jun 6;24(1):34.
8. Park Jae Mo, Reed Galen D, Liticker Jeff, Putnam William C, Chandra Alvin, Yaros Katarina, Afzal Aneela, MacNamara James P, Raza Jaffar, Hall Ronald G, Baxter Jeannie, Derner Kelley, Pena Salvador, Kallem Raja R, Subramanyan Indhumathy, Edpuganti Vindhya, Harrison Crystal, Muthukumar Alagar, Lewis Cheryl, Reddy Sangeetha, Unni Nisha, Klemow Dawn, Syed Samira, Li Hsiao-Ching, Cole Suzanne M, Froehlich Thomas, Ayers Colby R, de Lemos James A, Malloy Craig R, Haley Barbara, Zaha Vlad G. Effect of Doxorubicin on Myocardial Bicarbonate Production from Pyruvate Dehydrogenase in Women with Breast Cancer. Circulation Research [Internet]. American Heart Association; [cited 2020 Oct 21];0(0). Available from: https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.120.317970
9. Rider Oliver J, Apps Andrew, Miller Jack J, Lau Justin YC, Lewis Andrew JM, Peterzan Mark A, Dodd Michael S, Lau Angus Z, Trumper Claire, Gallagher Ferdia, Grist James T, Brindle Kevin, Neubauer Stefan, Tyler Damian J. Non-Invasive In Vivo Assessment of Cardiac Metabolism in the Healthy and Diabetic Human Heart Using Hyperpolarized 13C MRI. Circulation Research [Internet]. [cited 2020 Feb 10];0(0). Available from: https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.119.316260
10. Apps A, Lau JYC, Miller JJJJ, Tyler A, Young LAJ, Lewis AJM, Barnes G, Trumper C, Neubauer S, Rider OJ, Tyler DJ. Proof-of-Principle Demonstration of Direct Metabolic Imaging Following Myocardial Infarction Using Hyperpolarized 13C CMR. JACC Cardiovasc Imaging. 2021 Jun;14(6):1285–1288. PMCID: PMC8184499
11. Tang S, Milshteyn E, Reed G, Gordon J, Bok R, Zhu X, Zhu Z, Vigneron DB, Larson PEZ. A regional bolus tracking and real-time B1 calibration method for hyperpolarized 13 C MRI. Magn Reson Med. 2019 Feb;81(2):839–851. PMCID: PMC6289616
12. Tropp J, Lupo JM, Chen AP, Calderon P, McCune D, Grafendorfer T, Ozturk-Isik E, Larson PE, Hu S, Yen YF, Robb F, Bok R, Schulte R, Xu D, Hurd R, Vigneron D, Nelson S. Multi-Channel Metabolic Imaging, with SENSE reconstruction, of Hyperpolarized [1-13C] Pyruvate in a Live Rat at 3.0 tesla on a Clinical MR Scanner. J Magn Reson. 2011 Jan;208(1):171–177.
13. Gordon JW, Autry AW, Tang S, Graham JY, Bok RA, Zhu X, Villanueva-Meyer JE, Li Y, Ohilger MA, Abraham MR, Xu D, Vigneron DB, Larson PEZ. A variable resolution approach for improved acquisition of hyperpolarized 13 C metabolic MRI. Magn Reson Med. 2020 Dec;84(6):2943–2952. PMCID: PMC7719570
14. Zierhut ML, Yen YF, Chen AP, Bok R, Albers MJ, Zhang V, Tropp J, Park I, Vigneron DB, Kurhanewicz J, Hurd RE, Nelson SJ. Kinetic modeling of hyperpolarized 13C1-pyruvate metabolism in normal rats and TRAMP mice. J Magn Reson. 2010 Jan;202(1):85–92. PMID: 19884027
15. Larson PEZ, Chen HY, Gordon JW, Korn N, Maidens J, Arcak M, Tang S, Criekinge M, Carvajal L, Mammoli D, Bok R, Aggarwal R, Ferrone M, Slater JB, Nelson SJ, Kurhanewicz J, Vigneron DB. Investigation of analysis methods for hyperpolarized 13C-pyruvate metabolic MRI in prostate cancer patients. NMR Biomed. 2018 Nov;31(11):e3997. PMID: 30230646
16. Hyperpolarized-MRI-Toolbox [Internet]. Available from: https://github.com/LarsonLab/hyperpolarized-mri-toolbox
17. Hill DK, Orton MR, Mariotti E, Boult JKR, Panek R, Jafar M, Parkes HG, Jamin Y, Miniotis MF, Al-Saffar NMS, Beloueche-Babari M, Robinson SP, Leach MO, Chung YL, Eykyn TR. Model free approach to kinetic analysis of real-time hyperpolarized 13C magnetic resonance spectroscopy data. PLoS One. 2013;8(9):e71996. PMID: 24023724
18. Brindle KM, Campbell ID, Simpson RJ. A 1H-NMR study of the activity expressed by lactate dehydrogenase in the human erythrocyte. Eur J Biochem. 1986 Jul;158(2):299–305. PMID: 3732272
19. Romijn JA, Chinkes DL, Schwarz JM, Wolfe RR. Lactate-pyruvate interconversion in blood: implications for in vivo tracer studies. Am J Physiol. 1994 Mar;266(3 Pt 1):E334-40.
20. Lau AZ, Chen AP, Cunningham CH. Cardiac metabolic imaging using hyperpolarized [1-13C]lactate as a substrate. bioRxiv. 2020 Dec 6;2020.12.04.412296.
21. Mayer D, Yen YF, Josan S, Park JM, Pfefferbaum A, Hurd RE, Spielman DM. Application of hyperpolarized [1-13C]lactate for the in vivo investigation of cardiac metabolism. NMR Biomed. 2012 Oct;25(10):1119–1124. PMCID: PMC3357452
22. Chen AP, Lau JYC, Alvares RDA, Cunningham CH. Using [1-(13) C]lactic acid for hyperpolarized (13) C MR cardiac studies. Magn Reson Med. 2015 Jun;73(6):2087–2093. PMID: 25046652
Figures