1425
Development of a double tuned 2H/31P whole-body birdcage transmit coil for 2H and 31P MR applications from head to toe at 7T1Center for Image Sciences, University Medical Center Utrecht, Utrecht, Netherlands
Synopsis
Keywords: Whole Body, Metabolism, Deuterium, Phosphorus
Deuterium (2H) and phosphorus (31P) MRS are complementary methods for evaluating tissue metabolism non-invasively in vivo. Combined 2H and 31P MRS would therefore be of interest for various applications. In this work, we developed a double tuned 2H/31P whole-body birdcage transmit coil for 7T, for 2H and 31P MRS with homogeneous excitation over a large field-of-view. The B1+ variation of the whole-body birdcage coil over a body-sized phantom was 14% for 2H and 25% for 31P. Using a two-channel 2H/31P prototype receive array, we obtained high-quality 2H and 31P 3D MRSI data in the brain, liver, and lower-leg muscles.
Introduction
Phosphorus (31P) and (recently introduced) deuterium (2H) MRS1,2 are powerful tools to assess tissue metabolism in vivo in health and disease. Both techniques are capable to detect aberrant metabolism in tumor tissue: Increased cell proliferation in tumors leads to an increased ratio of phosphomonoesters-to-phosphodiesters, detectable by 31P MRS3,4, whereas the Warburg effect results in an increased production of lactate in tumors, which can be quantified with 2H MRS after administration of deuterated glucose1. Therefore, the combined application of 2H and 31P MRS in humans yields complementary information and would be of interest at various locations in the human body, ranging from the head to the body and extremities. For this purpose, we developed a double tuned 2H/31P whole-body birdcage transmit coil for 7T, for 2H and 31P MRS with homogeneous excitation over a large field-of-view and with the increased sensitivity offered by ultra-high field. Here, we characterize the 2H and 31P B1+ fields of the whole-body birdcage transmit coil and demonstrate the feasibility of combined 2H and 31P 3D MRSI measurements of the brain, liver and lower leg, using a two-channel 2H/31P receive array prototype.Methods
A shielded, double tuned 2H/31P whole-body birdcage transmit coil with a diameter of 60cm and a length of 40cm, embedded in the outside of the patient tube (Futura, Heerhugowaard, the Netherlands) of the MRI system, was constructed (Figure 1). The coil was tuned to 45.7 and 120.6 MHz for 2H and 31P, respectively, at 7T, by using LCC traps on the end rings, between the rungs. Data was acquired on a 7T whole-body MR system (Philips Healthcare, Best, the Netherlands), using a two-channel 2H/31P receive array prototype with two transmit-receive fractioned 1H dipole antennas (WaveTronica, Utrecht, The Netherlands). The 2H/31P whole-body birdcage transmit coil was driven by a single-channel, 10kW RF amplifier for 2H (using a quad hybrid; AN8112, Analogic Corporation, Peabody, MA, USA) or a two-channel, 2×18kW RF amplifier for 31P (AN8137, Analogic Corporation, Peabody, MA, USA).2H and 31P B1+ maps were acquired on a body size phantom containing 3g/L NaCl, 43mM KH2PO4, and 0.54% D2O, using the actual flip angle imaging (AFI) method5 (3D gradient echo: FOV=360×480×360mm3, resolution=30×30×30mm3, 2H/31P: TE=2.5/1.03ms, TR1=50/50ms, TR2=700/1000ms, nominal flip angle=65°/60°, reference B1+=8µT/10µT, NSA=12/128). B1+ maps were reconstructed using QMRI tools6.
2H and 31P MRSI measurements of the brain, liver and lower leg muscles were performed on one healthy volunteer (female, 34 years) at natural abundance and at rest. The receive array was positioned at the posterior side of the brain and lower leg, and the anterior side of the liver. Image-based B0 shimming was performed and T1w reference images were acquired for planning the 2H and 31P MRSI acquisitions. For both nuclei, a 3D MRSI sequence was used with a block excitation pulse and Hamming-weighted k-space acquisition (acquisition parameters in Figure 2). 2H and 31P MRSI were sequentially acquired without repositioning of subject. MRSI data were reconstructed in MATLAB 2022a (The MathWorks Inc., Natick, MA). PCA denoising was applied before Roemer equal noise channel combination7,8.
Results
Figure 1 shows B1+ maps of the 2H/31P whole-body birdcage transmit coil for both frequencies. Over the whole phantom, the B1+ variation (SD/mean) was 14% for 2H and 25% for 31P.In vivo 2H and 31P 3D MRSI data of the brain, liver and lower leg are shown in Figures 3-5. Signal intensity decreased at larger distances to the receive elements, but for most of the brain, liver and lower leg clear signals could be detected for both 2H and 31P. The 2H spectra of the brain, liver and muscle showed a signal from natural abundance deuterated water, with additional signals from lipids for voxels close to adipose tissue. The muscle deuterated water signals showed residual quadrupolar couplings, depending on the muscle type9. In the 31P spectra, high-energy phosphates were detected in all three tissues, together with phosphomonoesters and/or phosphodiesters.
Discussion and Conclusion
In this study, we developed a double tuned whole-body birdcage transmit coil for 2H and 31P at 7T. The B1+ field for 2H was very homogeneous (14% variation), as expected for this frequency (45.7 MHz, i.e. similar to 1H frequency at 1T). For 31P (120.6 MHz), the B1+ field was more inhomogeneous (25% variation), but comparable to what has been observed for 1H whole-body coils at 3T10. Using a two-channel 2H/31P prototype receive array, we obtained high-quality 2H and 31P 3D MRSI data in brain, liver, and muscle tissue. Optimized receive setups can be developed for specific applications, to be used in conjunction with the whole-body birdcage transmit coil, as is common for 1H applications at clinical field strengths.In conclusion, the developed double tuned 2H/31P whole-body birdcage transmit coil for 7T allows the combined application of 2H and 31P MRSI throughout the human body, for simultaneous 3D mapping of glucose and energy metabolism and membrane turnover.
Acknowledgements
This work was funded by an HTSM grant from NWO TTW (project number 17134) and by a FET Innovation Launchpad grant from the EU (grant number 850488).
References
1- De Feyter, H. M.; Behar, K. L.; Corbin, Z. A.; Fulbright, R. K.; Brown, P. B.; McIntyre, S.; Nixon, T. W.; Rothman, D. L.; De Graaf, R. A. Deuterium Metabolic Imaging (DMI) for MRI-Based 3D Mapping of Metabolism in Vivo. Sci. Adv. 2018, 4 (8), 1–12. https://doi.org/10.1126/sciadv.aat7314.
2- Lu, M.; Zhu, X. H.; Zhang, Y.; Mateescu, G.; Chen, W. Quantitative Assessment of Brain Glucose Metabolic Rates Using in Vivo Deuterium Magnetic Resonance Spectroscopy. J. Cereb. Blood Flow Metab. 2017, 37 (11), 3518–3530. https://doi.org/10.1177/0271678X17706444.
3- Cox, I. J.; Bell, J. D.; Peden, C. J.; Iles, R. A.; Foster, C. S.; Watanapa, P.; Williamson, R. C. N. In Vivo and in Vitro 31P Magnetic Resonance Spectroscopy of Focal Hepatic Malignancies. NMR Biomed. 1992, 5 (3), 114–120. https://doi.org/10.1002/NBM.1940050303.
4- van der Kemp, W. J. M.; Stehouwer, B. L.; Luijten, P. R.; van den Bosch, M. A. A. J.; Klomp, D. W. J. Detection of Alterations in Membrane Metabolism during Neoadjuvant Chemotherapy in Patients with Breast Cancer Using Phosphorus Magnetic Resonance Spectroscopy at 7 Tesla. Springerplus 2014, 3 (1). https://doi.org/10.1186/2193-1801-3-634.
5- Yarnykh, V. L. Actual Flip-Angle Imaging in the Pulsed Steady State: A Method for Rapid Three-Dimensional Mapping of the Transmitted Radiofrequency Field. Magn. Reson. Med. 2007, 57 (1), 192–200. https://doi.org/10.1002/mrm.21120.
6- Froeling, M. QMRTools: A Mathematica Toolbox for Quantitative MRI Analysis. J. Open Source Softw. 2019, 4 (38), 1204. https://doi.org/10.21105/joss.01204.
7- Froeling, M.; Prompers, J. J.; Klomp, D. W. J.; van der Velden, T. A. PCA Denoising and Wiener Deconvolution of 31P 3D CSI Data to Enhance Effective SNR and Improve Point Spread Function. Magn. Reson. Med. 2021, 85 (6), 2992–3009. https://doi.org/10.1002/mrm.28654.
8- Roemer, P. B.; Edelstein, W. A.; Hayes, C. E.; Souza, S. P.; Mueller, O. M. The NMR Phased Array. Magn. Reson. Med. 1990, 16 (2), 192–225. https://doi.org/10.1002/mrm.1910160203.
9- Gursan, A.; Froeling, M.; Hendriks, A. D.; Welting, D.; Kentgens, A. P. M.; Klomp, D. W. J.; Prompers, J. J. Residual Quadrupolar Couplings Observed in 7 Tesla Deuterium MR Spectra of Skeletal Muscle. Magn. Reson. Med. 2022, 87 (3), 1165–1173. https://doi.org/10.1002/mrm.29053.
10- Sacolick, L. I.; Wiesinger, F.; Hancu, I.; Vogel, M. W. B1 Mapping by Bloch-Siegert Shift. Magn. Reson. Med. 2010, 63 (5), 1315–1322. https://doi.org/10.1002/mrm.22357.
Figures
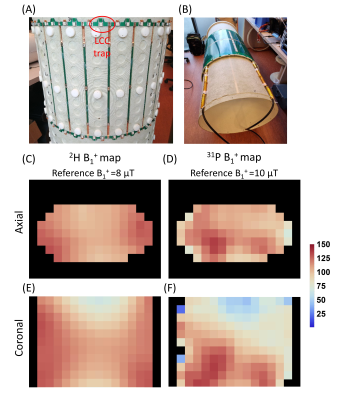
Figure 1. Developed double tuned 2H/31P whole-body birdcage transmit coil without (A) and with (B) shield, before installation in the 7T MRI system.2H (C,E) and 31P (D,F) AFI B1+ maps of the double tuned 2H/31P whole-body birdcage transmit coil measured on a body size phantom. Signals were received with a two-channel 2H/31P receive array placed on top of the phantom. Data are shown for a central transversal (C,D) and coronal (E,F) slice. The reference B1+ was 8 μT for 2H and 10 μT for 31P. The color bar indicates the percentage of this reference B1+.
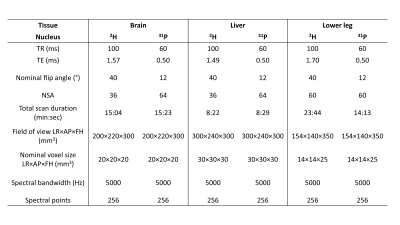
Figure 2. Acquisiton parameters for 2H and 31P 3D MRSI datasets collected in the brain, liver and lower leg. Field of view and nominal voxel size were identical for 2H and 31P scans of the same tissue.
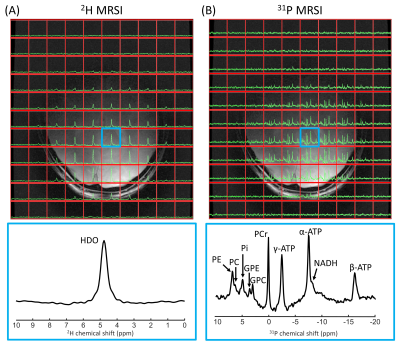
Figure 3. One slice of natural abundance 2H 3D MRSI (A) and 31P 3D MRSI (B) data of the brain overlaid on the T1w image, together with enlarged spectra from a selected voxel (indicated in blue). 2H/31P spectra were apodized with 20 Hz and zero-filled to 2048/512 points for visualization purposes. HDO, deuterated water; PE, phosphoethanolamine; PC, phosphocholine; Pi, inorganic phosphate; GPE, glycerophosphoethanolamine; GPC, glycerophosphocholine; PCr, phosphocreatine; NADH, nicotinamide adenine dinucleotide.
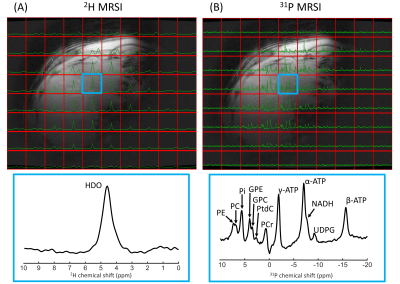
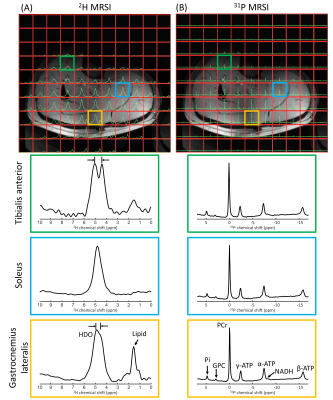
Figure 5. One slice of natural abundance 2H 3D MRSI (A) and 31P 3D MRSI (B) data of the lower leg overlaid on the T1w image, together with enlarged spectra from selected voxels in tibialis anterior, soleus and gastrocnemius lateralis (green, blue and orange). 31P spectra were apodized with 20 Hz and 2H/31P spectra were zero-filled to 2048/512 points for visualization purposes. The enlarged spectra for the different voxels were rescaled to equal signal intensity. In the tibialis and gastrocnemius 2H spectra, the deuterated water signal is split due to residual quadrupolar couplings9.