1420
MRF-based channel-wise absolute B1+ mapping at low RF power in the human abdomen at 7T1Physikalisch-Technische Bundesanstalt, Braunschweig and Berlin, Germany, 2Dept. of Radiology, Center for Biomedical Imaging, New York, NY, United States, 3Center for Advanced Imaging Innovation and Research, New York, NY, United States, 4School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 5Department of Biomedical Engineering, Technical University of Berlin, Berlin, Germany, 6Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 7Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States
Synopsis
Keywords: High-Field MRI, High-Field MRI
For pTx applications, knowledge of the underlying Tx channel-wise B1+ profile is essential. In this work, we present a hybrid absolute B1+-mapping approach where MRF-based B1+-mapping is utilized to map the channel-wise absolute B1+ profile, providing higher accuracy at low flip angles than standard methods. The hybrid approach is compared to mapping each Tx channel separately using the MRF-based method. Phantom and body in-vivo measurements at 7T show good agreement between the two approaches.
Introduction
The inhomogeneity of the transmit magnetic RF field (B1+) is one of the major challenges of imaging at ultra-high magnetic fields (≥7T), that can be compensated by parallel transmission(pTx)1. However, measuring complex B1+-profiles of each transmit(Tx) channel, which is a prerequisite to perform FA optimization, is highly challenging in the human abdomen at 7T not only due to strong B1+ variations. Furthermore, existing absolute B1+-mapping techniques for the body are limited in accuracy, especially at FAs below 20°.2 However, achieving high FAs is often difficult due to limited RF power and SAR limitations. To address these challenges, we recently demonstrated3, inspired by previous works4, a magnetic resonance fingerprinting (MRF)-based mapping technique that is highly promising for the human body since it accurately maps the B1+-field even at lower FAs. However, this approach only mapped the B1+-field for a channel-combined transmission case.In this work, we demonstrate the feasibility of accurate, high-resolution 2D channel-wise absolute B1+-mapping in the human abdomen at 7T with low RF power (8kW total for our system) using a hybrid B1+-mapping approach5,6 in under one minute acquisition time. The approach is evaluated experimentally at 7T in a phantom and in-vivo in a transversal slice of the liver and compared to mapping each channel separately using the MRF-based technique.
Methods
MRI was performed on a 7T scanner (Siemens Magnetom) using an 8Tx/16Rx body coil7. Hybrid mapping was performed by acquiring two MRF-based absolute B1+-maps with different RF-shim settings and one set of eight small tip-angle(STA) gradient-echo(GRE) images. For the GRE images only a single Tx channel was active at a time with all Rx channels active during breath-hold with Cartesian sampling using a zero-phase setting.For the MRF-based absolute B1+-mapping, a 2D radial FLASH sequence8 with time-varying FA-pattern9 (SINC-pulses,BWTP=4,duration=1.3ms) was applied during a breath-hold. 600 time-frames(TRs) and eight repetitions of the entire FA-pattern were acquired. Images were reconstructed using a non-uniform-FFT and pixel-wise matching to a dictionary. GRE data was used to obtain relative B1+-maps10 from which two complementary phase shims using AMORE11 were calculated. See Fig.1 for further details. As a reference, MRF was performed eight times with only one Tx-channel active.
Combiation of the channel-wise GRE-images and two absolute MRF B1+-maps was done in a procedure that follows previous hybrid approaches5,6 and is visualized in Fig.1/2. For each shim, a spatial calibration factor was calculated:
$$c_i(\vec{r})=\dfrac{|B_{1,i}^+(\vec{r})|}{|\sum_{Tx}GRE(Tx,\vec{r})\cdot\exp(-i\varphi_{i,Tx})|}\quad i\text{ (shim index)}=1,2\quad\text{and}\quad Tx=1,...,8$$
In a second step, $$$c_i(\vec{r})$$$ are weighted
$$C(\vec{r})=\dfrac{c_1(\vec{r})\cdot|B_{1,1}^+(\vec{r})|^2+c_2(\vec{r})\cdot|B_{1,2}^+(\vec{r})|^2}{|B_{1,1}^+(\vec{r})|^2+|B_{1,2}^+(\vec{r})|^2}$$
Then, the channel-wise absolute B1+-maps were obtained by:
$$B_{1,Tx}^+(\vec{r})=C(\vec{r})\cdot GRE(Tx,\vec{r})$$
The phase of $$$B_{1,Tx}^+$$$ was divided by $$$B_{1,Tx=1}^+$$$ to cancel out the receive phase. Outliers (steps>1µT/√kW to neighbouring pixels) were filtered out and given the mean values of their next neighbours.
Results & Discussion
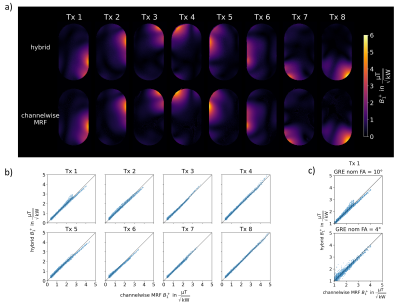
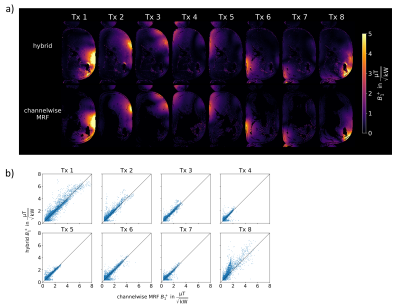
Fig.3 compares the hybrid approach to mapping each channel separately for a phantom. In a) qualitatively good agreement between the two approaches can be observed while b) shows the correlation between both methods, where considering all evaluated data points a Person correlation coefficient of 0.996 is observed (see figure caption for details).Fig.4 shows the in-vivo comparison in an axial liver slice. Qualitatively (a), the approaches agree, however, some deviations are visible close to the abdominal wall in Tx1/Tx8. b) shows the correlation, with a correlation coefficient of 0.928.
Deviations in the hybrid approach occur as adjacent to coil elements larger FAs violate the STA assumption (intensityGRE∝FA) and coil profiles overlap. For Tx1, Fig.3c shows this effect where the GREs were also acquired with lower nominal FA for the phantom. A higher correlation coefficient is observed for the GREs with larger nom FA over all data points of Tx1(10°:0.995, 4°:0.985), however, when only data points >2µT/√kW are considered, this reverses and the GRE acquisition with the lower flip angle shows higher correlation (4°:0.978, 10°:0.962). Consequently, accuracy could be improved using GRE acquisitions with multiple FAs12 at the cost of longer acquisition time.
In addition, deviations could occur due to different data sampling for MRF (radial) and GRE (Cartesian) acquisitions. The more extensive spread in the in-vivo data (Fig.4b vs 3b) could originate from multi-breath-hold acquisitions with shifts between the measurements. This could be improved by integrating all parts into one sequence.
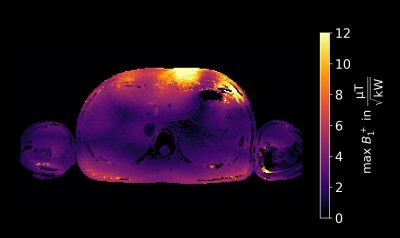
Fig.5 shows the maximum B1+ over the two shims. In the centre of the liver, multiple regions with values around 2µT/√kW and below are observed, pointing out that B1+-mapping approaches with reasonable accuracy at low FAs are essential such as the MRF approach utilized here if no dynamic shimming is applied, or more shim modes are considered.
Conclusion
We have demonstrated experimentally that high resolution channel-wise absolute B1+-mapping at low RF power is feasible with less than one minute acquisition time in the human abdomen at 7T. While our method still requires about a minute per slice, measuring the channel-wise absolute B1+-profile with high accuracy is for several applications more important than fast acquisition with lower accuracy, for example, for the design of universal pulse, for coil validation or for deep learning purposes where the accurate maps serve as training data. Furthermore, we were well below 50% 10s SAR, making 3D acquisitions conceivable. Thus, this work could be a useful tool for broader body MRI applications at 7T.Acknowledgements
We gratefully acknowledge funding from the German Research Foundation (GRK2260, BIOQIC and SCHM 2677/4-1). We thank Martijn Cloos, Centre for Advanced Imaging, University of Queensland for valuable discussions.References
1. Padormo F, Beqiri A, Hajnal JV, Malik SJ. Parallel transmission for ultrahigh-field imaging. NMR Biomed. 2016;29(9):1145-1161.
2. Pohmann R, Scheffler K. A theoretical and experimental comparison of different techniques for B1 mapping at very high fields. NMR Biomed. 2013;26(3):265-275.
3. Lutz M, Aigner CS, Dietrich S, et al. Low power free-breathing absolute B1+ mapping in the human body at 7T using magnetic resonance fingerprinting. In: Proceedings of the 30th Annual Meeting of ISMRM. 2022:0386.
4. Cloos MA, Paška J, Yu Z, et al. Abdominal Imaging with Heterogeneous Radiofrequency Fields at 7 Tesla. In: Proceedings of the 25th Annual Meeting of ISMRM. 2017:1125.
5. Brunheim S, Gratz M, Johst S, et al. Fast and accurate multi-channel mapping based on the TIAMO technique for 7T UHF body MRI. Magn Reson Med. 2018;79(5):2652-2664.
6. Van de Moortele PF, Snyder C, DelaBarre L, Adriany G, Vaughan T, Ugurbil K. Calibration Tools for RF Shim at Very High Field with Multiple Element RF Coils: From Ultra Fast Local Relative Phase to Absolute Magnitude B1+ Mapping. In: Proceedings of the 15th Annual Meeting of ISMRM. 2007:1676.
7. Ertürk MA, Raaijmakers AJE, Adriany G, Uğurbil K, Metzger GJ. A 16-channel combined loop-dipole transceiver array for 7 Tesla body MRI. Magn Reson Med. 2017;77(2):884-894.
8. Flassbeck S, Schmidt S, Bachert P, Ladd ME, Schmitter S. Flow MR fingerprinting. Magn Reson Med. 2019;81(4):2536-2550.
9. Jiang Y, Ma D, Seiberlich N, Gulani V, Griswold MA. MR fingerprinting using fast imaging with steady state precession (FISP) with spiral readout. Magn Reson Med. 2015;74(6):1621-1631.
10. Van de Moortele PF, Ugurbil K. Very Fast Multi Channel B1 Calibration at High Field in the Small Flip Angle Regime. In: Proceedings of the 17th Annual Meeting of ISMRM. 2009:367.
11. He X, Schmidt S, Zbýň Š, Haluptzok T, Moeller S, Metzger GJ. Improved TSE imaging at ultrahigh field using nonlocalized efficiency RF shimming and acquisition modes optimized for refocused echoes (AMORE). Magn Reson Med. 2022;88(4):1702-1719.
12. Padormo F, Hess AT, Aljabar P, et al. Large dynamic range relative B1+ mapping. Magn Reson Med. 2016;76(2):490-499
Figures
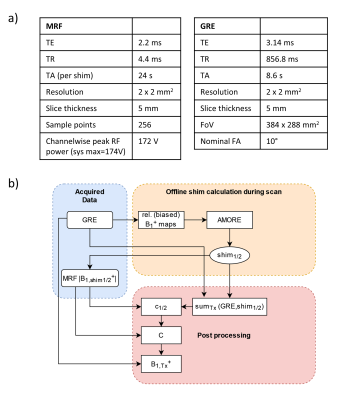
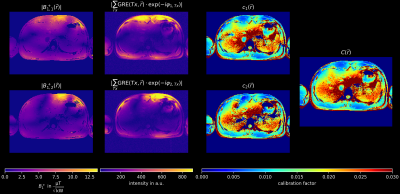
Figure 2: Intermediate results of the hybrid approach. Rows represent different phase shims (calculated using the AMORE cost function11). The first column shows the absolute B1+ maps measured using an MRF-based method. The same shim is applied to STA GRE images (column 2). Dividing column 1 by column 2 leads to the calibration factor ci (column 3). The calibration factors are then weighted based on the absolute B1+ value (column 1) to obtain the final calibration factor C (column 4).