1419
In-vivo 3D liver imaging at 7T using kT-point pTx pulses and a 32-Tx-channel whole-body RF antenna array1Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 2Faculty of Physics and Astronomy, Heidelberg University, Heidelberg, Germany, 3Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berlin, Germany, 4Institute of Radiology, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen-Nürnberg, Germany, 5Faculty of Medicine, Heidelberg University, Heidelberg, Germany, 6Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States
Synopsis
Keywords: High-Field MRI, Liver
Using a 32-Tx-channel whole-body RF antenna array at 7T could benefit exciting large body parts as these regions often suffer from flip angle inhomogeneity or dropouts. This study compares static pTx and 3D pTx pulses with a varying number of kT-points to the CP+ mode and non-optimized shim. With static pTx, the flip angle dropouts are reduced, and 2-3 kT-points seemed to deliver the best tradeoff between flip angle homogeneity and RF power.
Introduction
A main challenge in UHF body MRI are flip angle (FA) variations or voids, which arise from the spatially inhomogeneous B1+ transmit profiles of the RF antennas. To address this issue, static parallel transmit (pTx) (B1+ phase/magnitude shimming) has been applied with either local transmit (Tx) body arrays or remote Tx arrays,1,2 located behind the bore liner like body coils of clinical 1.5T/3T scanners. The latter enables a higher channel-count, resulting in a larger field of excitation than most local transmit arrays and provide superior patient comfort. However, when aiming at larger body regions (e.g. the liver), 7T studies from various groups using local arrays with 8 or 16 Tx channels showed that static pTx provides insufficient FA homogeneity3,4 and that dynamic pTx may be needed (e.g. spokes3,5, kT-points4), even when targeting a single slice.3 Thus, the question arises whether more than 16 Tx-channels and the more considerable distance to the body provided by a 32-Tx-channel whole-body antenna array2 benefits the 3D FA homogenization of a large target. This study demonstrates the application of static pTx and 3D dynamic kT-point pTx pulses for the whole liver in multiple subjects using a 32-Tx-channel whole-body array.Methods
Measurements were performed on a 7T MRI (Magnetom 7T, Siemens Healthcare, Germany), with a 32-channel antenna array integrated into the scanner bore.2 According to previous works6,7 relative 3D B1+maps of the liver were obtained in free-breathing using a radial phase-encoding (RPE) acquisition performed in 11min25s in 3 subjects (2m/1f, BMI=23.7-27.1 kg/m²). The parameters for the scans are listed in Figure 1. The B1+ magnitude and phase maps of an example transversal slice for one subject are shown in Figure 2. From these B1+ maps, 1 to 6 kT-point RF pulses were calculated in the small tip angle approximation with interleaved greedy and local optimization methods8,9 for each of the three subjects to excite an ROI covering the volume of the liver with a nominal 10° FA.7 For each kT-point, the regularization parameter is iterated between 10-6 and 10³, trading between RF power and excitation fidelity. Each optimization was carried out with 100 random starting phases, and L-curves were generated, displaying the RF power vs. the coefficient of variation (CV) of the FA within the ROI. Using these RF pulses, 3D GRE scans were acquired with up to 3 kT-points for Subject 1 and 2. Additionally, the default shim with magnitude 1 and phase 0 for all channels and a standard circularly polarized (CP+) mode were obtained.Results and Discussion
L-curve plots for 1-6 kT-points are shown in Figure 3. One kT-point (B1+ phase/magnitude shimming) provides CV values below 20% in two subjects, which typically indicates images without dropouts, as recently reported for the heart volume10 at 7T and as confirmed in Figure 4a. Subject 3 (highest BMI) shows a CV of 23.7% and a FA dropout close to the boundary of the ROI (Fig. 4a red arrow). Applying two kT-points results in a 1.5-to-2-fold reduction in CV and no observable FA dropouts in any subject for the same RF power as for a single kT-point. A further but less pronounced decrease in CV, which is also qualitatively visible from the FA homogeneity in Figure 4b, is obtained when further increasing the number of kT-points while maintaining the same RF power. As an alternative to reducing the CV, the RF power can be reduced 3-4 fold with two kT-points vs. one kT-point for the same CV as for one kT-point. 2-3 kT-points seem beneficial mainly for the normal BMI subjects when simultaneously aiming for low CV and low transmit power (grey arrow in Figure 3). This is because the blip gradients require an increasing fraction of the 1 ms fixed pulse duration for a growing number of kT-points, which leads to increased power. Figure 5 shows the in-vivo GRE images (receive profile not removed) of an example slice for the non-optimized shim, CP+ mode and one to three kT-points which match with their corresponding predictions. The FA dropouts are clearly seen for the non-optimized shim and the CP+ mode. These dropouts are removed using one kT-point in both subjects. Two and three kT-points improve the FA homogeneity further in both the predictions and the corresponding GRE scans. However, in these GRE scans, small FA variations are still visible, which might be caused by ΔB0 as it was not included in the design yet.Conclusion
With a 32-channel array, the preliminary data indicates that static pTx of the liver volume yields sufficient FA homogeneity in normal-weight subjects. Although further studies with more subjects are needed, 2-3 kT-points seem a practical solution that provides a good compromise between transmit power and FA fidelity for liver applications in the low FA regime for all subjects.Acknowledgements
No acknowledgement found.References
1. Vaughan, J.T., Snyder, C.J., DelaBarre, L.J., Bolan, P.J., Tian, J., Bolinger, L., Adriany, G., Andersen, P., Strupp, J. and Ugurbil, K. (2009), Whole-body imaging at 7T: Preliminary results. Magn. Reson. Med., 61: 244-248. https://doi.org/10.1002/mrm.21751.
2. Orzada S, Solbach K, Gratz M, Brunheim S, Fiedler TM, Johst S, Bitz AK, Shooshtary S, Abuelhaija A, Voelker MN, Rietsch SHG, Kraff O, Maderwald S, Flöser M, Oehmigen M, Quick HH, Ladd ME. A 32-channel parallel transmit system add-on for 7T MRI. PLoS One. 2019 Sep 12;14(9):e0222452. doi: 10.1371/journal.pone.0222452. PMID: 31513637; PMCID: PMC6742215.
3. Wu X, Schmitter S, Auerbach EJ, Uğurbil K, Van de Moortele PF. Mitigating transmit B 1 inhomogeneity in the liver at 7T using multi-spoke parallel transmit RF pulse design. Quant Imaging Med Surg. 2014 Feb;4(1):4-10. doi: 10.3978/j.issn.2223-4292.2014.02.06. PMID: 24649429; PMCID: PMC3947979.
4. Runderkamp B., van der Zwaag W., Roos T., Strijkers G., Caan M., Nederveen A. Whole-liver flip angle shimming at 7T using eight-channel parallel transmission kt-points pulses with FPE-DREAM B1+ mapping. Proc. ISMRM (2022) #2868.
5. Shirvani S., Ding B., Dragonu I., Liebig P., Rua C., Karkouri J., Klomp D., Hess A. T., Rodgers C. T. Initial experience on 7T Terra for human parallel transmit (pTx) liver imaging. Proc. ISMRM (2020) #4288.
6. Dietrich S, Aigner CS, Kolbitsch C, Mayer J, Ludwig J, Schmidt S, Schaeffter T, Schmitter S. 3D Free-breathing multichannel absolute B1+ Mapping in the human body at 7T. Magn Reson Med. 2021 May;85(5):2552-2567. doi: 10.1002/mrm.28602. Epub 2020 Dec 7. PMID: 33283915.
7. Aigner, Christoph & Dietrich, Sebastian & Schmitter, Sebastian. (2020). Three-dimensional static and dynamic parallel transmission of the human heart at 7 T. NMR in Biomedicine. 34. 10.1002/nbm.4450.
8. Grissom, W.A., Khalighi, M.-M., Sacolick, L.I., Rutt, B.K. and Vogel, M.W. (2012), Small-tip-angle spokes pulse design using interleaved greedy and local optimization methods. Magn Reson Med, 68: 1553-1562. https://doi.org/10.1002/mrm.24165.
9. Cao Z, Yan X, Grissom WA. Array-compressed parallel transmit pulse design. Magn Reson Med. 2016 Oct;76(4):1158-69. doi: 10.1002/mrm.26020. Epub 2015 Oct 28. PMID: 26510117; PMCID: PMC4848238.
10. Aigner CS, Dietrich S, Schaeffter T, Schmitter S. Calibration-free pTx of the human heart at 7T via 3D universal pulses. Magn Reson Med. 2022 Jan;87(1):70-84. doi: 10.1002/mrm.28952. Epub 2021 Aug 16. PMID: 34399002.
Figures

Figure 1: a) Scan parameters for the relative B1+ maps and the GRE scans with non-opt. shim, CP+mode, static pTx and 2 and 3 kT-points. b) Example kT-points pulse train with 3 kT-points for subject 1 with a total puls duration of 1ms and a blip duration of 80µs.
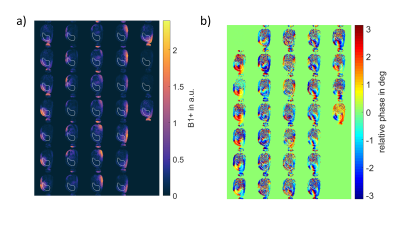
Figure 2: Relative B1+ maps of one example slice through the liver for all 32 channels showing a) magnitude and b) relative phase of the channels with respect to Channel 1. The ROI used for optimization is marked in white.

Figure 3: RMS RF power vs. FA CV for a) male, BMI: 23.66 kg/m² b) female, BMI: 25.93 kg/m² c) male, BMI: 27.13 kg/m². Grey arrow: best tradeoff between RF power and FA CV.

Figure 4: FA predictions for an example transversal slice through the liver with a) 1kT-point for all subjects and b) of Subject 1 with non-optimized shim and 1 to 6 kT-points.

Figure 5: Magnitude images and FA predictions of an example transversal slice through the liver for non-optimized shim, CP+ mode and 1-3 kT-points for Subject 1 and Subject 2. The optimization ROI is marked in white in the prediction maps.