1417
Optimized T1 and T2 Weighted Structural Imaging on Clinical 7 Tesla Systems Employing Single Channel GRAPE Universal Pulses
Eberhard Daniel Pracht1, Daniel Löwen1, Laurent Lamalle2, Franck Mauconduit3, Vincent Gras3, Nicolas Boulant3, and Tony Stöcker1,4
1German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany, 2GIGA-CRC in Vivo Imaging, University of Liège, Liège, Belgium, 3Université Paris-Saclay, Commissariat à l’Energie Atomique, CNRS, NeuroSpin, BAOBAB, Paris-Saclay, France, 4Department of Physics and Astronomy, University of Bonn, Bonn, Germany
1German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany, 2GIGA-CRC in Vivo Imaging, University of Liège, Liège, Belgium, 3Université Paris-Saclay, Commissariat à l’Energie Atomique, CNRS, NeuroSpin, BAOBAB, Paris-Saclay, France, 4Department of Physics and Astronomy, University of Bonn, Bonn, Germany
Synopsis
Keywords: High-Field MRI, Brain
We present single channel universal GRAPE pulses for optimized T1 and T2 weighted (fluid suppressed) imaging on clinical ultra-high field systems. B0 and B1 inhomogeneities are effectively mitigated while no pulse calculations are necessary during the imaging session. Compared to a parallel transmission set-up SAR estimation is less complex, and with more established history of safe use, it makes this approach a promising tool for clinical applications such as lesion detection.Introduction
In the last years, ultra-high field (7T) imaging has become an increasingly important modality for neuroscience applications. However, various issues compromise image quality and scanning efficiency at UHF, e.g. B1 and B0 inhomogeneities induce signal drop outs and/or insufficient suppression of unwanted tissue components, and SAR limitations reduce scanning efficiency. The use of parallel transmission (pTX) mitigates these issues efficiently. By applying universal pulses, calibration scans and online pulse calculations become unnecessary [1]. So far, pTX is restricted to research settings and is not yet applicable for clinical use. To overcome this, we propose single channel universal pulses (sTX-UPs, [2]) for clinical ultra-high field applications. We designed and implemented non-selective excitation, inversion, as well as refocusing pulses to facilitate the acquisition of all standard image contrasts for structural imaging (T1w, T2w, T2w-FLAIR).Methods
Single channel universal pulses were calculated to achieve uniform excitation, refocusing, and inversion patterns over the whole brain by utilizing the GRAPE algorithm [3, 4] and minimizing the flip angle NRMSE. The optimization was initialized with SPINS pulses [5]. All pulses were designed under explicit power constraints and with simultaneous optimization of the k‐space trajectory under slew rate constraints. The optimization was based on B0 and B1+ data derived from a population of healthy adults in a pre-study, rendering RF calibration procedures unnecessary during the actual imaging session.For T1w (MPRAGE) contrast an excitation pulse (small tip angle approximation, nominal FA=5deg), and an inversion pulse (high flip angle regime, nominal FA=180deg) were calculated.For T2w (SPACE) contrast a scalable RF pulse [5] with nominal flip angle of 90deg was calculated. The pulse was used for excitation and refocusing within the variable flip angle train. The excitation pulse phase was shifted by 90deg relative to the refocusing pulses to fulfill the CPMG condition. For fluid suppression a universal pulse T2prep module was used [6]. All refocusing pulses were implemented using a symmetric RF shape and k-space trajectory to fulfill the CPMG condition. This was achieved by concatenating two excitation pulses (Figure 1).Imaging experiments were performed on a MAGNETOM 7TPlus and a FDA approved (CE labeled) 7T Terra scanner (Siemens AG, Healthcare Sector, Erlangen, Germany) using standard head array coils with one transmit and 32 receive channels (Nova Medical, Wilmington, USA). For pulse performance analysis and comparison to a parallel transmission set-up, additional experiments were performed with 8 transmit channels. Imaging was performed in two healthy volunteers. Imaging parameters: 0.8x0.8x0.8mm resolution, matrix size 320x320x208 (whole brain coverage). MPRAGE: TR/TI = 2500/1100ms, total scan time 4:45 min. FLAIR sequence parameters: TR = 9000ms, TI/TE = 2100/300ms, echo spacing CP/pTX-UP/sTX-UP = 3.28/3.44/7.75ms, total scan time 5:09 min.
Results
Figure 2 shows a Bloch simulation for the 90deg SPACE refocusing pulse. The flip angle homogeneity of the sTX-UP is similar to the pTX-UP, however at the cost of a longer pulse duration (2.7ms vs. 0.76ms) due to the loss of the coil channels degrees of freedom. Figures 3+4 show MPRAGE and FLAIR images acquired with CP-mode, sTX-UP, and pTX-UP pulses. Due to the B1 insensitivity of universal pulses, no artifacts originating from incomplete inversion or refocusing are visible, and B0 as well as B1 inhomogeneities are effectively mitigated. As can be seen in Fig. 3+4 (bottom right) even down to the cerebellum high signal level, as well as effective inversion can be maintained and the images appear significantly more homogeneous over the brain compared to the CP-mode acquisition. The average NRMSE was comparable to the pTX-UPs (approximately 10% for all sTX-UP pulses, except for the inversion pulse were a NRMSE of approximately 6% was achieved). A slight SNR and CNR penalty can be observed for all sTX-UP images due to the increased pulse durations, as non-optimized standard protocols have been used so far for all acquisitions. Figure 5 shows the imaging protocols acquired in "Clinical Mode" using a single channel transmit coil.Discussion and Conclusion
Substantial improvement of image excitation, refocusing, and inversion homogeneity over the whole brain was achieved utilizing sTX-UPs and GRAPE. Future work will include SAR comparisons as well as optimization of the imaging protocols with regard to SNR and CNR, in order to account for the longer pulse durations of the sTX-UP pulses. Additionally, the pulse performance will be further optimized by adapting pulse duration and energy to the updated protocol needs.Acknowledgements
This work received financial support from the European Union Horizon 2020 Research and Innovation program under grant agreement 885876 (AROMA). Furthermore, the work is part of the SCAIFIELD project under the aegis of JPND, supported through the following funding organizations: Belgium, The Fund for Scientific Research (F.R.S.-FNRS); Germany, Federal Ministry of Education and Research (BMBF; funding codes 01ED2109A/B); Norway, The Research Council of Norway (RCN); Turkey, Scientific and Technological Research Council of Turkey, TÜBİTAK.References
[1] Gras et al. Magn Reson Med. 2017;77,635-642
[2] Mooiweer et al. Magn Reson Imag. 2022;92,182-186
[3] Khaneja N. et al. J. Magn. Reson. 2005; 172:296-305
[4] van Damme et al. Magn Reson Med. 2020;85,678-693
[5] Malik et al. Magn Reson Med. 2012;67,1303–1315
[6] Gras et al. Magn Reson Med. 2018;80,53-65
[7] Gras et al. Magn Reson Med. 2019;81,3202-3208
Figures
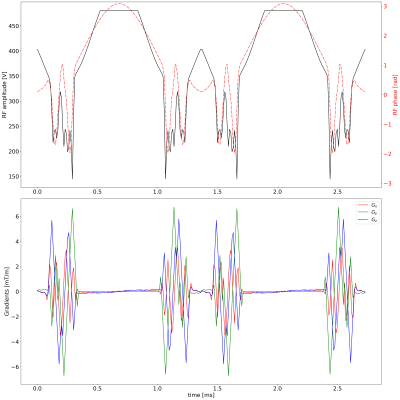
Figure1: 180deg refocusing pulse of the T2prep module. The pulse is created by concatenating two 90deg excitation pulses. The pulse symmetry is needed to achieve phase coherence over the whole brain volume between excitation and refocusing. The first half of the pulse is used for excitation (by adding a constant 90deg phase shift).
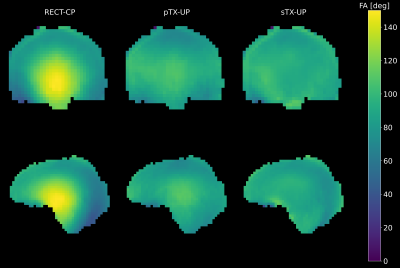
Figure 2: Bloch simulation for the SPACE excitation pulse. Left: Standard rectangular CP-mode excitation. Middle: pTX-UP excitation. Right: sTX-UP excitation. Both, sTX-UP and pTX-UP achieve a homogeneous flip angle over the whole brain volume. However, for the sTX-UP at cost of a long pulse duration due to the loss of the channel degree of freedom compared to the pTX-UP.
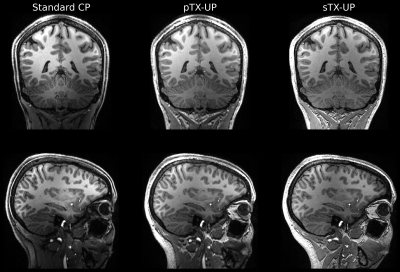
Figure 3: Comparison of MPRAGE imaging protocols: Left: Standard adiabatic inversion and rectangular CP-mode excitation. Middle: pTX-UPs for both, inversion and excitation. Right: sTX-UP application for excitation and inversion. In comparison to the CP mode acquisition, B0 and B1 inhomogeneities are mitigated for the universal pulse acquisition.
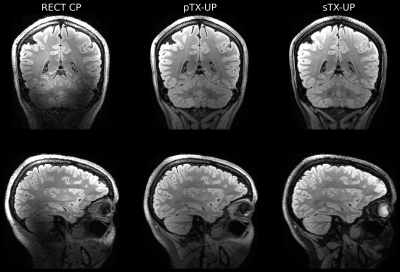
Figure 4: Comparison of FLAIR imaging protocols: Left: Standard CP-mode protocol: Signal drop-outs in the Cerebellum are clearly visible. Middle, right: The universal pulses generate an homogeneous FLAIR contrast over the field of view. Even down to the cerebellum full inversion is maintained. The sTX-UP achieves a slightly better homogeneity, but less SNR due to the longer refocusing pulses.
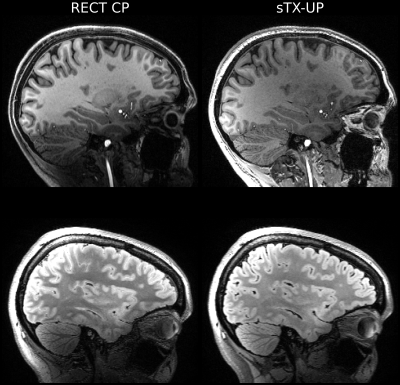
Figure 5: Universal pulse acquisition in "Clinical Mode": sTX-UPs with GRAPE outperform the standard CP-mode acquisition. B0 and B1 inhomogeneities are effectively mitigated.
DOI: https://doi.org/10.58530/2023/1417