1416
Improvement of Brain MRS at 7T Using a Wireless RF Array1Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Synopsis
Keywords: High-Field MRI, Spectroscopy
Magnetic resonance spectroscopy (MRS) can particularly benefit from substantial enhancement in SNR and spectral resolution at ultra-high field (UHF ≥7T), enabling improved quantification of metabolites. However, at 7T wavelength effects cause a highly inhomogeneous transmit magnetic field in the human brain, with lower transmit efficiency in the posterior-fossa manifesting as signal dropout in this region. Recently, we reported advantages of a surface-applied inductively-coupled radiofrequency array to improve transmit efficiency and signal sensitivity at 7T MRI focusing on cerebellum and inferior temporal lobes. Here we demonstrate the feasibility and effectiveness of in-vivo MRS using the array in human cerebellum at 7T.Introduction
Ultra-high field (UHF ≥7T) systems provide improved sensitivity and resolution over conventional field strengths1, enabling robust in-vivo application of MRS techniques. However, implementing MRS in the human brain at UHF introduces challenges such as transmit magnetic field (B1) inhomogeneity due to wavelength effects2. The human cerebellum plays an important role in the functional activity of the brain, ranging from motor to cognitive systems. However, the caudal location and complex structure of the cerebellum render posterior fossa challenging for MRS3, specifically at UHF, due to reduced transmit efficiency in this region, causing a significantly reduced SNR compared with other brain areas. Approaches to resolving issues of inhomogeneity include parallel transmit systems (PTx)4, the design of specialized RF pulses5, and passive RF shimming techniques such as high dielectric materials6 or metasurfaces7. However, these methods have some drawbacks such as unstable material parameters of dielectric pads, high cost and complexity of PTx systems, and complex structure of the metasurfaces. Recently, a simple approach based on a wireless passive radiofrequency (RF) array has been proposed by our group8. The array is easily placed against the posterior and caudal portion of the head inside a head coil, that markedly improves regional transmit efficiency and signal sensitivity. The aim of this study is to assess the effect of this new wireless RF array technology for enhancing in-vivo MRS in human cerebellum at 7T.Methods
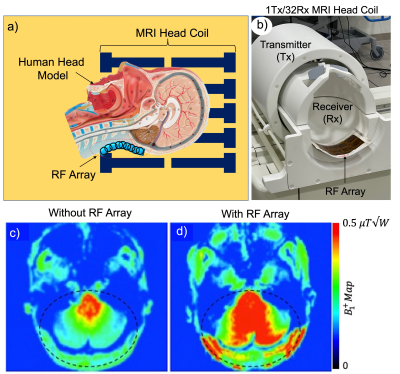
The phantom and in-vivo experiments were performed on a Siemens 7T whole-body human scanner (Magnetom, Siemens Healthcare, Erlangen, Germany) with a Nova 1Tx/32Rx head coil (Nova Medical, Wilmington, MA, USA). The wireless array was placed in the inferior position behind the neck inside the head coil overlying the posterior fossa, where the transmit efficiency is inherently low (Fig. 1a). Figure 1b shows the prototype of the RF array and the position of the array relative to the head coil. The experimental protocol was consisted of paired acquisition of data sets: with and without the RF array for mapping, structural imaging, and MRS comparisons. In-vivo B1 maps were measured using the presaturation-prepared turbo-FLASH based method9. Anatomical in-vivo images were acquired using T2-TSE sequences with 0.4 mm2 in-plane resolution. For the phantom and in-vivo MRS, the semi-LASER sequence was implemented using a non-adiabatic frequency modulated 90° excitation pulse. All data were acquired with TR/TE = 3000/32 ms. A water suppression sequence was used in conjunction with a semi-LASER sequence to acquire water-suppressed metabolite spectra. Prior to data acquisition, a field-map-based shimming routine was used to optimize field homogeneity, and the RF transmitter power was calibrated using a localized optimization routine. For in-vivo MRS, a volume of interest (VOI) in the cerebellum was defined based on T1-weighted images. MR metabolite spectra were analyzed with LCmodel10 using a simulated set of metabolites as the basis set and water signal as reference. The analysis was carried out over a chemical shift range of 0.2–4.2 ppm.Results
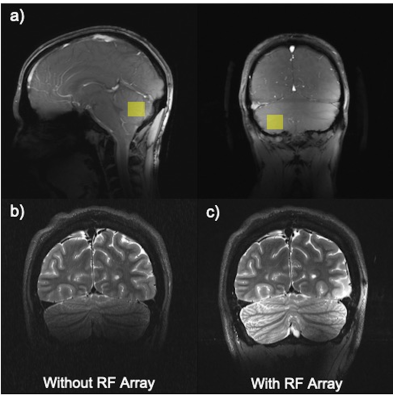
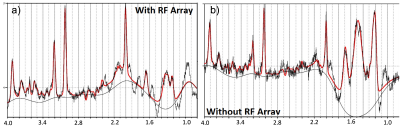
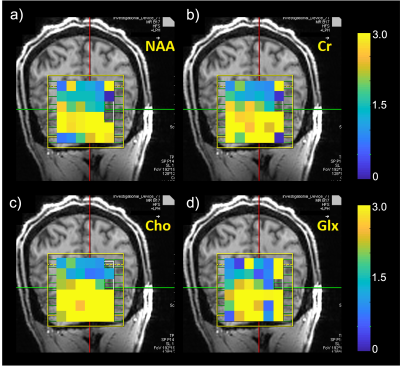
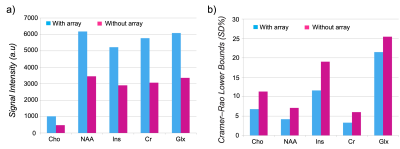
The spatial distribution of the B1 maps in the posterior fossa shows a mean 2-fold improvement within a region-of-interest (ROI) when the array is present (Fig. 1c, d). Figure 2a shows the sagittal and coronal planes from the Localizer scan through the cerebellum. The VOI position is indicated by the box. In-vivo imaging results of human brain in the coronal plane with/without the array are shown in Figure 2b,c. Qualitative comparison of the images shows that the array results in a large improvement in the image uniformity, SNR, and tissue contrast in the cerebellum. Comparison of MRS plot in presence vs. absence of the array demonstrated average SNR enhancement of a factor of 2.1 and 1.9 in the phantom and in-vivo (Fig. 3a, b), respectively. Figure 4 shows the gain of the signal intensity of each metabolite plotted in a 6x6 grid with the corresponding enhanced location. In-vivo MRS SNR gain was 1.9-fold for n-acetylaspartate (NAA, Fig. 4a), 1.7-fold for Creatine (Cr, Fig. 4b), 2.2-fold for choline (Cho, Fig. 4c), and 1.8-fold for glutamate+glutamine (Glx, Fig. 4d). The relatively uniform gain also meant metabolite ratios weren’t significantly altered with and without the array. Figure 5a, b shows the estimated standard deviations of the metabolite concentrations expressed as Cramer–Rao lower bounds (CRLBs), calculated in both with and without the array in vivo. Results show 37% reduction in CRLBs, indicating more confident fits. Results in phantom showed comparable performance, 35% reduction in CRLBs.Discussion and Conclusion
In this study, we used a wireless RF array to improve MRS at 7T. The overall results indicate that the localized MRS has been improved significantly in the presence of the array. The array can significantly increase detection sensitivity and may reduce the RF transmission power and data acquisition time for MRS and MRI applications specifically at 7T which MRS needs a high-power RF pulse. The proposed passive RF array could provide a cost-effective and efficient solution to improve detection sensitivity for human MRS and MRI in the regions with lower transmit efficiency.Acknowledgements
This work was supported by an NIH grant (NIH R01CA202911). All studies involving human subjects were performed in accordance with the MRI safety review board and institutional review board of the Icahn School of Medicine at Mount Sinai.References
[1] Godlewska BR, Clare S, Cowen PJ, Emir UE. Ultra-High-Field Magnetic Resonance Spectroscopy in Psychiatry. Front Psychiatry. 2017 Jul 11;8:123.
[2] Foo, T. K., Hayes, C. & Kang, Y. W. Reduction of RF penetration effects in high field imaging. Magn. Reson. Med. 23, 287–301(1992).
[3] Emir UE, Sood J, Chiew M, Thomas MA, Lane SP. High-resolution metabolic mapping of the cerebellum using 2D zoom magnetic resonance spectroscopic imaging. Magn Reson Med. 2021 May;85(5):2349-2358.
[4] Katscher U, B.P. Parallel RF transmission in MRI. NMR Biomed 19, 8 (2006).
[5] B. M. Gras V, Vignaud A, Ferrand G, Amadon A, Mauconduit F, et al, "Homogeneous non-selective and slice-selective parallel-transmit excitations at 7 Tesla with universal pulses: A validation study on two commercial RF coil," PLoS ONE vol. 12, no. 8, p. e0183562, 2017.
[6] M. W. Yang QX, Wang J, et al, "Manipulation of image intensity distribution at 7.0 T: passive RF shimming and focusing with dielectric materials," J Magn Reson Imaging, vol. 24, no. 1, pp. 197‐202, 2006, doi: 10.1002/jmri.20603.
[7] R. Schmidt, A. Slobozhanyuk, P. Belov, and A. Webb, "Flexible and compact hybrid metasurfaces for enhanced ultra-high field in vivo magnetic resonance imaging," Scientific Reports, vol. 7, no. 1, p. 1678, 2017/05/10 2017, doi: 10.1038/s41598-017-01932-9.
[8] A. Alipour et al., "Enhanced Ultra-High Field Brain MRI Using a Wireless Radiofrequency Sheet," International Society for Magnetic Resonance in Medicine, 2021.
[9] Klose, U. (1992), Mapping of the radio frequency magnetic field with a MR snapshot FLASH technique. Med. Phys., 19: 1099-1104.
[10] Provencher, S.W. (2001), Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed., 14: 260-264.
Figures