1411
Phantom validation of continuous radial sampling MRI for robotic interventional cardiovascular surgery1School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China, 2Institute of Medical Robotics, Shanghai Jiao Tong University, Shanghai, China
Synopsis
Keywords: Vessels, Cardiovascular, Run-time MRI, Navigation, Interventional MRI
Cardiovascular dilation surgery is usually performed with DSA navigation, and excessive radiation during imaging harms both the patient and the physician. Magnetic resonance imaging can avoid ionizing radiation, but its imaging efficiency severely limits the performance of real-time imaging. Therefore, we construct a complete real-time magnetic resonance navigation system through continuous radial scanning mode and corresponding reconstruction methods and formulate a group of scanning strategies. The characteristics of the special derivative catheter under magnetic resonance imaging were tested by combining the partner's magnetically compatible robotic equipment. And the results demonstrated the feasibility of this method.Introduction
Balloon Dilatation (BD) is an effective surgery for stenosis of aorta1. Cardiovascular surgery is mostly realized through DSA image navigation2, which has a certain degree of radiation damage to patients and doctors. Continuous radial sampling MRI provides the feasibility of real-time imaging, which could be a promising substitution. Therefore, we construct a real-time monitoring image navigation system based on MRI3. Relying on the custom-defined reconstruction method provided by Gadgetron4, the sampling data can be processed and visualized run-time by the fast reconstruction method, then used to guide the behavior of surgical instruments. The whole process of cardiovascular dilatation surgery was simulated on a cardiovascular model, and the results of offline post-processing were compared to preliminarily verify the feasibility of this idea.Methods
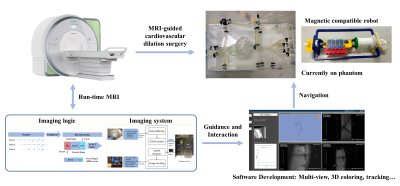
MRI scans are executed throughout the operation. With run-time processing of these data, intraoperative images are obtained and visualized for both the operator and the robot. Based on this information, the behavior of surgical instruments can be further guided to complete the entire surgical process, as shown in Fig 1.With the conventional cardiovascular dilatation surgery process executed with robots, three imaging methods were performed. 2D run-time MRI imaging on the arcus aortae plane with a big thickness (16mm) was carried out at a fast speed. A 2.5D strategy, multi-slice with different directions at one time, was performed for more information. And 3D imaging was used for a much slower operation like balloon dilatation. Also, a non-intercurrent non-gated in vivo cardiac imaging was executed for validation. The whole process was finished on a Siemens Aera 1.5T scanner (Siemens Healthineers, Erlangen, Germany).
Two contrast pulse sequences, gradient echo (GRE) and balanced steady-state free precession (bSSFP5) were employed to scan the target plane continuously for a long time with radial trajectory. By applying the directional evolution model of the golden angle strategy, the uniform distribution requirement of subsequent data reconstruction is reduced6. A multi-layer alternating continuous sampling method (2.5D strategy) and a stack of star trajectory (3D strategy) were further designed, to provide more information for the whole surgical process from multiple directions7.
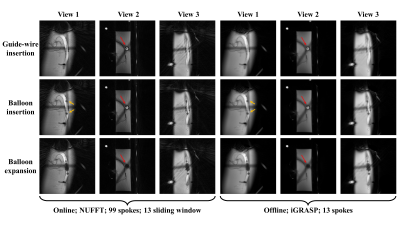
To adapt the sequence design and meet the navigation requirements, the reconstruction process was redesigned with Gadgetron software to establish the run-time reconstruction. The Non-Uniform Fast Fourier Transform (NUFFT) method is applied to the online reconstruction on a single GPU RTX 3090. In addition, the iGRASP model was applied to obtain the offline reconstructed image for comparative illustration8.
The robot system is a magnetic-compatible pneumatic cardiovascular dilatation surgery system designed by the IMR Team of Shanghai Jiao Tong University. The guide wire is doped with ferric oxide to introduce local magnetic field inhomogeneity and obtain hypointense signal characterization. The experiments were implemented on a conventional aortic arch model which is filled with the standard nickel sulfate solution, and the cardiac pulse was simulated by an external pump.
Results and Discussion
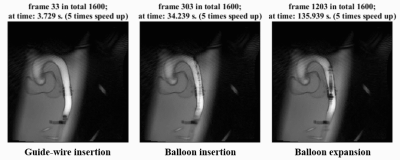
The 2D run-time imaging results are shown in Fig 2. The whole process of guide wire insertion, catheter insertion, balloon expansion, and instrument withdrawal can be effectively captured during surgery. Even with a large degree of data sharing in the reconstruction, there is no serious blur or residual image on the images, because the whole experimental operation process itself was relatively slow.Fig 3 shows the 2.5D results for both online and offline reconstruction. As viewed from different directions, the instruments’ behavior can be better tracked and lead to a decrease in time resolution, but for slow processes, it is a favorable tradeoff.
Fig 4 is the 3D imaging for balloon dilatation. Also, the process is clear and the balloon’s whole moving process can be observed. The problem is that mass data size for one frame leads to a significantly delayed visualization, which would be risky in practical application.
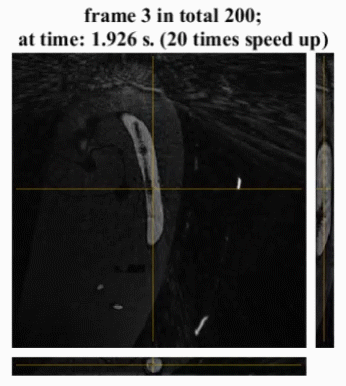
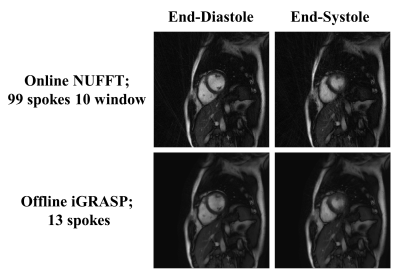
The in vivo cardiac imaging shows the potential of our method for practical use. As shown in Fig 5, the heartbeat is distinguishable but with blurring, especially at the end-systole phase. Offline reconstruction reduces the artifacts but also blurs the image.
Conclusions
The consistency of online and offline reconstructions indicates the feasibility of using magnetic resonance imaging to guide cardiovascular surgery. Improvement will be carried out for better imaging in future investigations.Acknowledgements
This work is supported by the National Natural Science Foundation of China National Science Foundation of China (No. 62001290), Shanghai Science and Technology Development Funds (21DZ1100300), and sponsored by the National Science and Technology Innovation 2030 Major Project (2022ZD0208601).References
1. Tworetzky W, Wilkins-Haug L, Jennings RW, et al. Balloon dilation of severe aortic stenosis in the fetus: potential for prevention of hypoplastic left heart syndrome: Candidate selection, technique, and results of successful intervention. Circulation 2004;110:2125–2131.
2. Heijenbrok-Kal MH, Kock MCJM, Hunink MGM. Lower extremity arterial disease: Multidetector CT angiography—Meta-Analysis. Radiology 2007;245:433–439.
3. Winkelmann S, Schaeffter T, Koehler T, Eggers H, Doessel O. An optimal radial profile order based on the golden ratio for time-resolved MRI. IEEE Trans. Med. Imaging 2007;26:68–76.
4. Hansen MS, Sørensen TS. Gadgetron: An open source framework for medical image reconstruction: Gadgetron. Magn. Reson. Med. 2013;69:1768–1776.
5. Deshpande VS, Chung Y-C, Zhang Q, Shea SM, Li D. Reduction of transient signal oscillations in true-FISP using a linear flip angle series magnetization preparation. Magn. Reson. Med. 2003;49:151–157.
6. Feng L. Golden‐Angle radial MRI: Basics, advances, and applications. J. Magn. Reson. Imaging 2022;56:45–62.
7. Block KT, Chandarana H, Milla S, et al. Towards routine clinical use of radial stack-of-stars 3D gradient-echo sequences for reducing motion sensitivity. J. Korean Soc. Magn. Reson. Med. 2014;18:87.
8. Feng L, Grimm R, Block KT, et al. Golden-angle radial sparse parallel MRI: Combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI: iGRASP: Iterative Golden-Angle RAdial Sparse Parallel MRI. Magn. Reson. Med. 2014;72:707–717.
Figures