1407
Respiratory Motion-Resolved 4D Pulmonary Imaging with Stack-of-Spiral UTE at 0.55T and 3T1Siemens Medical Solutions USA, Inc., Malvern, PA, United States, 2MR Application Predevelopment, Siemens Healthcare GmbH, Erlangen, Germany, 3Cardiovascular Branch, Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, United States
Synopsis
Keywords: Lung, Low-Field MRI
Recently, ultrashort echo time (UTE) has gained renewed interest for lung imaging at 1.5T and 3T, which captures short T2* signal from lung parenchyma. Lower field is attractive for lung MRI due to prolonged T2* and reduced susceptibility, and it was demonstrated that high-resolution high-quality structural lung imaging can be achieved with UTE at 0.55T. In addition to anatomical information, pulmonary function such as regional ventilation is also of great clinical interest. In this study, we developed free-breathing respiratory-motion-resolved 4D pulmonary imaging using stack-of-spirals acquisition with compressed-sensing at both 0.55T and 3T, which could enable quantitative evaluation of ventilation dynamics.INTRODUCTION
Lung MRI is challenging due to low proton density, short T2*, and susceptibility gradients at tissue-air interfaces. Recently, ultrashort echo time (UTE) has gained renewed interest for lung imaging at 1.5T and 3T, which captures short T2* signal from lung parenchyma1. Lower field is attractive for lung MRI due to prolonged T2* and reduced susceptibility2, and it was demonstrated that high-resolution high-quality structural lung imaging can be achieved with UTE at 0.55T3. In addition to anatomical information, pulmonary function such as regional ventilation is also of great clinical interest particularly in patients with chronic lung diseases such as cystic fibrosis and chronic obstructive pulmonary disease (COPD)4. In this study, we developed free-breathing respiratory-motion-resolved 4D pulmonary imaging using stack-of-spirals acquisition with compressed sensing reconstruction at both 0.55T and 3T, which could enable quantitative evaluation of ventilation dynamics.METHODS
Self-gated Stack-of-Spiral UTE:Data were acquired in coronal orientation with a Spiral VIBE UTE research application sequence on a 0.55T and a 3T scanner (MAGNETOM Free.Max and 3T MAGNETOM Vida; Siemens Healthcare, Erlangen, Germany). A total of 3 healthy volunteers were scanned during free-breathing with informed consent (one at 0.55T and two at 3T). 3D k-space is acquired with stack-of-spirals and partition-in-line ordering, using spiral sampling with golden angle reordering in-plane and Cartesian sampling along the partition direction. Non-selective RF pulses combined with adaptive TE along the slice encoding direction enable short TE5. Superoinferior (SI) navigators are applied throughout the scan for monitoring respiratory signal. No external gating or triggering was used. Imaging parameters: 1) 0.55T: acquisition time = 8min, resolution = 2x2x2mm3, FOV = 450x450x260mm3, TE/TR = 0.05/8.5msec; FA = 8°; spiral arms = 350; readout length = 5.4msec; gating temporal resolution = 247msec; 2) 3T: acquisition time = 8min, resolution = 1.5x1.5x1.5mm3, FOV = 480x480x249mm3, TE/TR = 0.05/3.7msec, FA = 5°, spiral arms = 700; readout length = 1.7msec; gating temporal resolution = 127msec.
Gating signal analysis and binning:
Respiratory motion signal was extracted by retrospectively processing the acquired gating signal using principal component analysis (PCA). Based on the amplitude of the gating signal, the acquired data is uniformly binned into five respiratory states, where each state contains a subset of the data and spiral trajectories.
Motion-resolved compressed sensing reconstruction:
Compressed Sensing is used to exploit sparsity along the respiratory dimension to obtain motion-resolved images from different respiratory phases. Images across phases are treated as a 3D tensor, and redundant Haar wavelets6 are used to constrain sparsity in both spatial and respiratory directions. For the data fidelity term, an iterative density compensation is used7. The compressed sensing iterative reconstruction was performed using FISTA algorithm. Reconstruction is written in C++ and is deployed in-line on the 0.55T and 3T scanners.
RESULTS
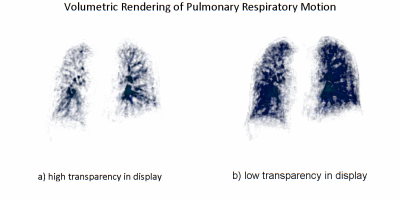
Figure 1 shows plot of the extracted gating signal (duration of 100 sec) and corresponding binning results of five respiratory phases based on signal amplitude. Figure 2 shows respiratory-motion-resolved images from a volunteer at 3T. Five bins correspond to five respiratory motion states from end-expiration to end-inspiration. Red arrows (axial view) and red horizonal lines (coronal & sagittal views) show the movement of the diaphragm during respiration. Figure 3 shows respiratory motion resolved images with five phases from a volunteer at 0.55T. Improved delineation of the lung anatomy, increased parenchymal signal, and reduced blurring can be observed in each respiratory phase compared to 3T, due to reduced susceptibility and prolonged T2* at lower field. The video in Figure 4 captures the respiratory motion of 3D lung (volumetric rendering) across five respiratory phases with data acquired at 0.55T (same as Fig. 3), displayed with two different levels of transparency. Figure 4a with high transparency delineates more vessel details, while Figure 4b shows the volumetric movement of the lung tissue.DISCUSSION and CONCLUSION
In this work, free-breathing respiratory-motion-resolved 4D pulmonary imaging at both 0.55T and 3T is demonstrated by leveraging compressed sensing reconstruction in combination with stack-of-spirals sampling. We used a 2mm isotropic resolution at 0.55T and 1.5mm isotropic resolution at 3T (both at 8 minute acquisition), and additional comparisons are warranted. Besides morphological imaging, this technique can be promising for evaluation of pulmonary function such as ventilation dynamics, using the methods such as 3D phase-resolved functional lung imaging (PREFUL)8.Acknowledgements
No acknowledgement found.References
[1] Mugler JP, Meyer CH, Pfeuffer J, et al. Accelerated Stack-of-Spirals Breath-hold UTE Lung Imaging. ISMRM 2017:4904.
[2] Campbell-Washburn AE, Ramasawmy R, Restivo MC, et al. Opportunities in Interventional and Diagnostic Imaging by Using High-Performance Low-Field-Strength MRI. Radiology. 2019;293(2):384-393. doi:10.1148/radiol.2019190452
[3] Javed A, Ramasawmy R, O’Brien K, et al. Self-gated 3D stack-of-spirals UTE pulmonary imaging at 0.55T. Magn Reson Med. 2022; 87: 1784– 1798. doi:10.1002/mrm.29079
[4] Feng L, Delacoste S, et al. (2019), Simultaneous Evaluation of Lung Anatomy and Ventilation Using 4D Respiratory-Motion-Resolved Ultrashort Echo Time Sparse MRI. J. Magn. Reson. Imaging, 49: 411-422. https://doi.org/10.1002/jmri.26245
[5] Qian Y, Boada FE. Acquisition-weighted stack of spirals for fast high-resolution three-dimensional ultra-short echo time MR imaging. Magn Reson Med. 2008 Jul;60(1):135-45. doi: 10.1002/mrm.21620. PMID: 18581326.
[6] Liu J, Rapin JC, et al. Dynamic cardiac MRI reconstruction with weighted redundant Haar wavelets. ISMRM 2012: 4249.
[7] Pipe JG and Menon P. (1999), Sampling density compensation in MRI: Rationale and an iterative numerical solution. Magn. Reson. Med., 41: 179-186. https://doi.org/10.1002/(SICI)1522-2594(199901)41:1<179::AID-MRM25>3.0.CO;2-V
[8] Klimeš F, Voskrebenzev A, Gutberlet M, et al. 3D phase-resolved functional lung ventilation MR imaging in healthy volunteers and patients with chronic pulmonary disease. Magn Reson Med. 2020; 85: 912– 925. https://doi.org/10.1002/mrm.28482
Figures