1403
Iron Oxide Nanoparticle MR Lymphangiography (ION-MRL): A quantitative technique for assessing the peripheral and central lymphatic systems.1Radiology, Stanford, Stanford, CA, United States
Synopsis
Keywords: Vessels, Quantitative Imaging, lymphangiography
A lack of clinical quantitative lymphatic metrics slows progress for innovation in treatment of lymphatic disease. MR lymphangiography provides high resolution anatomic analysis of the lymphatic system necessary for guiding new and emerging therapeutics but has not been explored as a quantitative metric. We coupled MR lymphangiography with iron oxide nanoparticles as the contrast agent and demonstrate the time to visualization of the thoracic duct is decreased in patients with bilateral lymphedema. Additionally, the change in R2* of inguinal lymph nodes after transpedal contrast injection quantifies lymphatic transit in patients with lymphedema.Introduction
New and emerging therapeutic options for patient are increasingly relying on MR lymphangiography (MRL) for diagnosis and management guidance1–3. Compared to the clinically available small molecule gadolinium contrast agents, the iron oxide nanoparticle ferumoxytol is a more appropriate size (17-31 nm)4 for lymphatic imaging (similar in size to the agents utilized for lymphoscintigraphy). Unlike the gadolinium agents, after subcutaneous injection ferumoxytol cannot readily diffuse into the blood vasculature due to its size, removing the need for vascular contamination suppression techniques. Additionally, once ferumoxytol is taken into the lymphatic system, it cannot readily diffuse out, allowing contrast to be tracked more proximally including through the central lymphatics5. A possible additional advantage of utilizing ferumoxytol as a lymphatic contrast agent, is that R2* based methods have been developed to allow quantitation of iron-oxide nanoparticles over large concentration ranges thereby providing the opportunity for lymphatic contrast concentration quantification6–9. In this project, we explore the utility of iron oxide nanoparticle (ION)-MRL in imaging the lymphatic system, the timing of ferumoxytol contrast transit into the thoracic duct, and the use of R2* in quantifying lymphatic contrast concentration in lymphatic disease.Methods
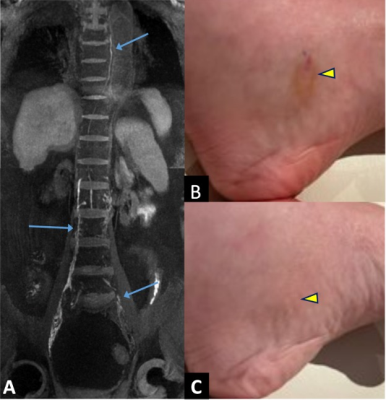
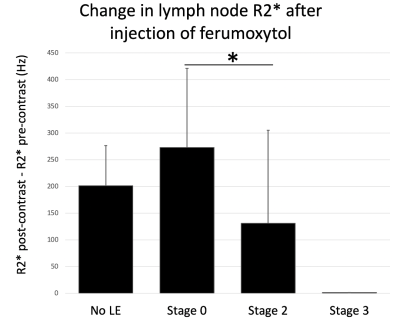
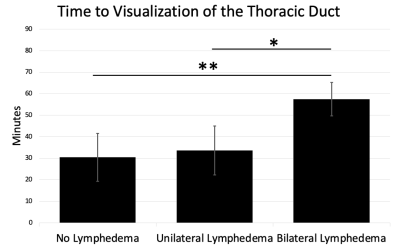
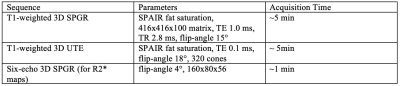
A retrospective analysis of 27 patients (14 male, 13 female) who underwent ION-MRL as part of routine clinical care was performed with institutional review board (IRB) approval and waiver of informed consent. MRI utilized a 3T scanner (GE Signa Premier, GE Signal Architect, or GE 750w) with two anterior arrays (2x 30-channel GE AIR coils, or vascular run-off coil plus 16-channel torso coil), and a 60-channel bed embedded posterior array. Sequences were similar to Table 1. Patients were counseled regarding the expected skin discoloration due to ferumoxytol (see Figure 1). Ferumoxytol use was monitored per FDA guidelines for 30-min following injection, utilizing MRI compatible blood pressure cuffs and cardiac leads. A dose of 32-40 mg of ferumoxytol diluted to 16-20 mL in saline was split into 10 subcutaneous injections (5 injections per foot), with 1-min of massage applied to injection sites. Injections were given at the first and second interweb space, the anterolateral foot, the posterolateral foot and inferior to the medial malleolus, to highlight the 4 major lymphosomes of the leg10. Patients were then ambulated to promote muscular activity of the lower extremities. Two radiologists, with 16 years and 6 years of radiology experience, and both with experience in lymphatic imaging, evaluated images. To evaluate the time from injection to visualization of the thoracic duct in ION-MRL, studies that included imaging the area of the thoracic duct were evaluated (n=20). Patients were classified (no lymphedema, unilateral lymphedema, bilateral lymphedema) based on clinical notes. Time from injection to first visualization was recorded. Two patients were excluded because the thoracic duct was not visualized during the 2-hour allotted exam time; both patients had bilateral lymphedema. To evaluate the concentration of lymphatic contrast transit in ION-MRL, studies that included R2* mapping of the pelvis before and after ferumoxytol injection were evaluated. Of 54 lower extremities evaluated with ION-MRL, 16 were excluded because R2* maps were not acquired in the pelvis, two were excluded because only the contralateral extremity was injected with ferumoxytol, and two were excluded because no inguinal lymph node was identified (both patients had stage 2 lymphedema). A region of interest was drawn encompassing the inguinal lymph node that demonstrated maximal enhancement, and the mean R2* (Hz) was recorded before and after contrast administration. Each lower extremity was characterized as no lymphedema, stage 1 lymphedema, stage 2 lymphedema, or stage 3 lymphedema based on the international society of lymphology staging system. Patients with unilateral lymphedema were given a stage 0 for the contralateral limb.Results/Discussion
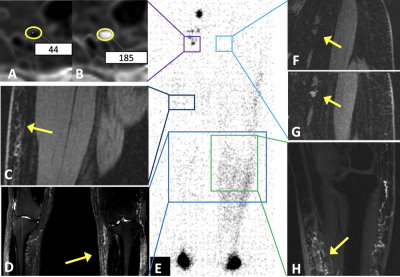
Two illustrative case studies of ION-MRL are presented (Figures 1 & 2). One challenge in treating lymphatic disease is the lack of quantitative tools to characterize the lymphatic system. In this project, we show that quantitation of lymphatic contrast concentration with R2* maps of inguinal lymph nodes was able to significantly differentiate stage 0 and stage 2 lymphedema (Figure 3). Of note, a wide standard deviation was noted in the patients with stage 2 lymphedema. The quantitative difference in lymphatic transit suggests that this method could be used to differentiate patients within clinical stage categories. We also show the average time to visualization of the thoracic duct in patients without lower extremity lymphedema receiving ION-MRL to be ~30-min (Figure 4). This timing parameter was significantly increased in patients with bilateral lymphedema compared to patients with unilateral lymphedema and no lymphedema. As quantitative metrics of lymphatic function have been demonstrated to predict surgical outcomes of lymphedema surgeries11, ION-MRL could provide not only anatomical characterization but also quantitative parameters for guidance of therapeutic management for patients with lymphatic disease. Limitations of this study include a relatively small study population, and that imaging was not optimized for evaluating these proposed quantitative measures. Future studies will benefit from utilizing fixed timing of image sequence acquisition relative to contrast injection time.Conclusion
ION-MRL provides anatomical depiction of peripheral and central lymphatic anatomy with two quantitative metrics that differentiate between lymphatic disease states.Acknowledgements
No acknowledgement found.References
1. Salehi BP, Sibley RC, Friedman R, et al. MRI of Lymphedema. Journal of Magnetic Resonance Imaging. n/a(n/a). doi:10.1002/jmri.28496
2. Neligan PC, Kung TA, Maki JH. MR lymphangiography in the treatment of lymphedema. Journal of Surgical Oncology. 2017;115(1):18-22. doi:10.1002/jso.24337
3. MRI of the Central Lymphatic System: Indications, Imaging Technique, and Pre-Procedural Planning. Accessed September 9, 2021. https://oce.ovid.com/article/00002142-201708000-00005/PDF
4. DailyMed - FERAHEME- ferumoxytol injection. Accessed September 24, 2021. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=32b0e320-a739-11dc-a704-0002a5d5c51b
5. Maki JH, Neligan PC, Briller N, Mitsumori LM, Wilson GJ. Dark Blood Magnetic Resonance Lymphangiography Using Dual-Agent Relaxivity Contrast (DARC-MRL): A Novel Method Combining Gadolinium and Iron Contrast Agents. Current Problems in Diagnostic Radiology. 2016;45(3):174-179. doi:10.1067/j.cpradiol.2015.08.003
6. Liu W, Dahnke H, Rahmer J, Jordan EK, Frank JA. Ultrashort T2* relaxometry for quantitation of highly concentrated superparamagnetic iron oxide (SPIO) nanoparticle labeled cells. Magn Reson Med. 2009;61(4):761-766. doi:10.1002/mrm.21923
7. Magnitsky S, Zhang J, Idiyatullin D, et al. Positive contrast from cells labeled with iron oxide nanoparticles: Quantitation of imaging data. Magn Reson Med. 2017;78(5):1900-1910. doi:10.1002/mrm.26585
8. Sharma SD, Fischer R, Schoennagel BP, et al. MRI-based quantitative susceptibility mapping (QSM) and R2* mapping of liver iron overload: Comparison with SQUID-based biomagnetic liver susceptometry. Magn Reson Med. 2017;78(1):264-270. doi:10.1002/mrm.26358
9. Vasanawala SS, Yu H, Shimakawa A, Jeng M, Brittain JH. Estimation of liver T₂ in transfusion-related iron overload in patients with weighted least squares T₂ IDEAL. Magn Reson Med. 2012;67(1):183-190. doi:10.1002/mrm.22986
10. Shinaoka A, Koshimune S, Suami H, et al. Lower-Limb Lymphatic Drainage Pathways and Lymph Nodes: A CT Lymphangiography Cadaver Study. Radiology. 2020;294(1):223-229. doi:10.1148/radiol.2019191169
11. Association of lymphatic flow velocity with surgical outcomes in patients undergoing lymphovenous anastomosis for breast cancer-related lymphedema | SpringerLink. Accessed November 5, 2022. https://link.springer.com/article/10.1007/s12282-022-01363-z
Figures