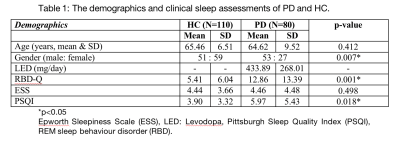
1389
Non-motor Correlates of Pedunculopontine Nucleus Projection Denervation in Parkinson’s Disease with Sleep Disturbances1Diagnostic Radiology, Singapore General Hospital, Singapore, Singapore, 2Graduate Institute of Medicine, Yuan-Ze University, Taoyuan City, Taiwan, 3Department of Neurology, National Neuroscience Institute, Singapore, Singapore, 4Radiography, Singapore General Hospital, Singapore, Singapore
Synopsis
Keywords: Parkinson's Disease, Diffusion/other diffusion imaging techniques
The mechanism by which involvement of the cholinergic pathways underlies the non-motor manifestations of Parkinsonian disorders remains unclear. We mapped and investigated the pedunculopontine nucleus (PPN) projections that exhibit significant correlations with sleep disturbances in 80 Parkinson’s disease (PD) patients and 110 healthy controls (HC) using correlational tractography. Intracellular and extracellular diffusivity (RDI and NRDI metrics respectively) demonstrated significant correlations (r=0.06-0.14, FDR<0.05) with sleep abnormalities in the PPN projections of PD compared to HC. Our findings established non-motor markers of PPN system denervation with sleep disturbances to further understand the pathophysiology of non-motor symptoms in PD.Introduction
Motor impairment is the classic presentation in Parkinson’s disease (PD), however, non-motor symptoms such as changes in mood and behavior, cognitive impairment, sleep disorders, and olfactory dysfunction also co-occur in the early stages of PD1,2. Sleep disturbances may precede the clinical manifestations of motor symptoms3,4 and the symptoms include insomnia, restlessness, nocturnal psychosis, nocturia, excessive daytime sleepiness (EDS), restless legs syndrome, periodic limb movements of sleep, rapid eye movement sleep behavior disorder (RBD), and obstructive sleep apnea5. Cholinergic systems degeneration is associated with impaired cognition, falling, slower gait speed, RBD, and impaired olfaction whereas the noncholinergic neurons are more directly involved in motor functions 1,6. Non-motor symptoms may reflect cholinergic system degeneration in PD that may affect pedunculopontine nucleus (PPN)-thalamic projections7,8. The PPN controls muscle tone during wakefulness and is responsible for atonia during REM sleep9, with vast interconnections to hypothalamic and brainstem regions involved in the regulation of the sleep-wake cycle via thalamocortical activation and behavioral states. Unfortunately, the mechanism by which the involvement of the cholinergic pathways, particularly PPN projections underlies the non-motor manifestations of parkinsonian disorders remains unclear. In addition, symptoms including gait deficits and sleep disorders are unresponsive to L-DOPA treatment, providing the rationale for exploring the heterogeneity of cholinergic system degeneration in PD.Methods
Institutional ethics board approval and patient informed consent were obtained for this study. Eighty PD patients and 110 healthy controls (HC) underwent brain MRI using diffusion spectrum imaging (DSI) on a 3T scanner (Skyra, Siemens Healthcare, Erlangen, Germany). The DSI imaging parameters were as follows: TR/TE=4100/110 ms, in-plane resolution = 2x2x2 mm3, diffusion sampling= 129 directions, and maximum b-value = 3000 s/mm2. The clinical non-motor assessments characterizing sleep disturbances, such as the REM Sleep Behavior Disorder Screening Questionnaire (RBDSQ), Pittsburgh Sleep Quality Index (PSQI), and Epworth Sleepiness Scale (ESS) were carried out for all participants. Whole brain correlational tractography10-13 was performed using connectometry analysis in DSI studio (http://dsi-studio.labsolver.org) with PPN as a seed to map the PPN projections exhibiting significant correlations with sleep abnormalities. The false discovery rate (FDR) threshold was 0.05 and age and gender were included as covariates. The restricted (RDI) and non-restricted (NRDI) diffusion imaging metrics were sampled along the PPN projection that established significant correlations with sleep assessments to quantify the intracellular and extracellular diffusivity respectively14. The Mann-Whitney test was carried out to establish group differences in the diffusion metrics of the PPN projections between PD and HC groups.Results
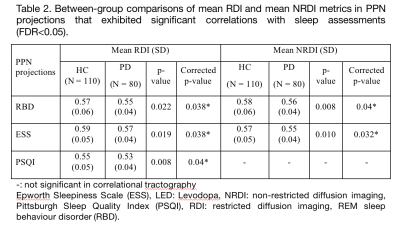
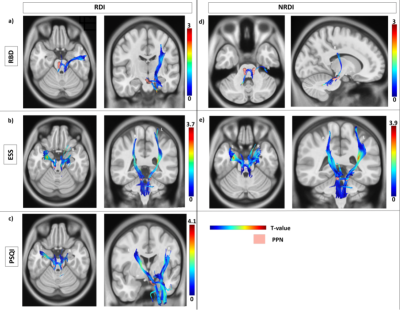
The subject demographics are tabulated in Table 1. The findings from the correlational tractography demonstrated significant positive correlations between RDI (r=0.06-0.14, FDR<0.05) with ESS, PSQI, and RBD; and NRDI (r=0.09-0.13, FDR<0.05) with ESS and RBD in the PPN projections (Figure 1). The tract profiles of the PPN bundles correlated to sleep disturbances showed significant differences in diffusion metrics with sleep assessments between PD and HC (Table 2). Decreased RDI and NRDI were seen in PPN projections that were correlated with RBD (mean RDIPD: 0.55, mean RDIHC:0.57, p= 0.038 and mean NRDIPD: 0.56, mean NRDIHC:0.58, p= 0.04) and ESS (mean RDIPD: 0.57, mean RDIHC: 0.59, p= 0.038 and mean NRDIPD: 0.55, mean NRDIHC: 0.57, p= 0.032) in PD compared to HC. Reduced RDI was also observed in PPN projection correlated with PSQI (mean RDIPD: 0.53, mean RDIHC: 0.55, p= 0.04). The distributions of the diffusion metrics and sleep assessments were presented in Figure 2.Discussion
Our findings showed the loss of integrity of affected PPN projections that exhibited significant correlations to sleep disturbances. These axonal bundles correlated to RDI with all three sleep assessments and NRDI with PSQI were parts of the dentorubrothalamic, reticular, corticospinal, and corticobulbar tracts. The projections correlated to NRDI with RBD overlapped with the reticular and corticopontine tracts. The reduced intracellular and extracellular diffusivity (RDI and NRDI metrics) with sleep assessments in the cholinergic neurotransmission of the PD cohort could be contributed by reduced axonal density and less myelination. Decreased connectivity has been reported in PD with EDS patients in the left and right fornix, left and right inferior longitudinal fasciculus, left inferior fronto-occipital fasciculus, left inferior, body of the corpus callosum, splenium, left corticospinal tract, right cingulum, and middle cerebellar peduncles in comparison to PD non-EDS group and HC 3,4. Neocortical, limbic cortical and thalamic cholinergic denervation in PD patients with RBD using PET study15. The data on cholinergic system degeneration in PD with sleep disorders have been limited or heterogeneous.Conclusion
Correlational tractography is a sensitive and robust method for mapping axonal PPN projections exhibiting significant correlations with sleep abnormalities. The loss of integrity of affected PPN projections correlated to sleep disturbances was seen in PD with reduced intracellular and extracellular diffusivity.Acknowledgements
We thank the National Medical Research Council, Singapore, our funding agency, andexcellent teams of MR radiographers and research coordinators at the Department of16Diagnostic Radiology, Singapore General Hospital, for their kind assistance and support inthis study.References
1. Bergamino M, Keeling EG, Mishra VR, Stokes AM, Walsh RR. Assessing White Matter Pathology in Early-Stage Parkinson Disease Using Diffusion MRI: A Systematic Review. Front Neurol. 2020;11:314.
2. Postuma RB, Bertrand JA, Montplaisir J, et al. Rapid eye movement sleep behavior disorder and risk of dementia in Parkinson's disease: a prospective study. Movement disorders. 2012;27(6):720-726.
3. Gama RL, Távora DG, Bomfim RC, Silva CE, de Bruin VM, de Bruin PF. Sleep disturbances and brain MRI morphometry in Parkinson's disease, multiple system atrophy and progressive supranuclear palsy–a comparative study. Parkinsonism & related disorders. 2010;16(4):275-279.
4. Ashraf-Ganjouei A, Kheiri G, Masoudi M, et al. White matter tract alterations in drug-naive Parkinson's disease patients with excessive daytime sleepiness. Frontiers in neurology. 2019;10:378
5. Morita H, Hass CJ, Moro E, Sudhyadhom A, Kumar R, Okun MS. Pedunculopontine Nucleus Stimulation: Where are We Now and What Needs to be Done to Move the Field Forward? Frontiers in Neurology. 2014;5(243).
6. Müller ML, Bohnen NI, Kotagal V, et al. Clinical markers for identifying cholinergic deficits in Parkinson's disease. Movement Disorders. 2015;30(2):269-273.
7. Bohnen NI, Müller ML, Dauer WT, Albin RL. Parkinson’s disease: what role do pedunculopontine cholinergic neurons play? Future neurology. 2014;9(1):5-8.
8. Bohnen NI, Müller ML, Kotagal V, et al. Heterogeneity of cholinergic denervation in Parkinson's disease without dementia. Journal of Cerebral Blood Flow & Metabolism. 2012;32(8):1609-1617.
9. Benarroch EE. Pedunculopontine nucleus: functional organization and clinical implications. Neurology. 2013;80(12):1148-1155.
10. Yeh FC, Badre D, Verstynen T. Connectometry: A statistical approach harnessing the analytical potential of the local connectome. NeuroImage. 2016;125:162-171.
11. Yeh FC, Panesar S, Barrios J, et al. Automatic Removal of False Connections in Diffusion MRI Tractography Using Topology-Informed Pruning (TIP). Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2019;16(1):52-58.
12. Yeh FC, Tseng WY. NTU-90: a high angular resolution brain atlas constructed by q-space diffeomorphic reconstruction. NeuroImage. 2011;58(1):91-99.
13. Yeh FC, Wedeen VJ, Tseng WY. Generalized q-sampling imaging. IEEE Trans Med Imaging. 2010;29(9):1626-1635.
14. Yeh F-C, Liu L, Hitchens TK, Wu YL. Mapping immune cell infiltration using restricted diffusion MRI. Magnetic resonance in medicine. 2017;77(2):603-612.
15. Kotagal V, Albin RL, Müller MLTM, et al. Symptoms of rapid eye movement sleep behavior disorder are associated with cholinergic denervation in Parkinson disease. Ann Neurol. 2012;71(4):560-568.
Figures