1387
Enzyme Delivery to the Putamen in Parkinson’s Disease Patients by MR-Guided Focused Ultrasound1Physical Sciences, Sunnybrook Research Institute, Toronto, ON, Canada, 2Division of Neurosurgery, Sunnybrook Health Sciences Centre, Toronto, ON, Canada, 3Hurvitz Brain Sciences Research Program, Sunnybrook Research Institute, Toronto, ON, Canada, 4Department of Medicine, University of Toronto, Toronto, ON, Canada, 5Krembil Research Institute, University Health Network, Toronto, ON, Canada, 6Division of Neurosurgery, Toronto Western Hospital, Toronto, ON, Canada, 7Division of Neurology, Toronto Western Hospital, Toronto, ON, Canada, 8Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada, 9Institute of Biomedical Engineering, University of Toronto, Toronto, ON, Canada
Synopsis
Keywords: Parkinson's Disease, Focused Ultrasound
The phase I clinical trial demonstrated the successful application of microbubble-assisted MR-guided Focused Ultrasound for blood-brain barrier (BBB) opening in the putamen to facilitate biweekly therapeutic drug delivery in patients with Parkinson's disease. BBB permeability within the targeted putamen was elevated successfully in all treatments, as revealed by Gd-enhanced T1-weighted MRI immediately post treatment. No contrast enhancement was observed in the treated putamen on MR imaging scans acquired one day following each treatment session, indicating closure of the BBB. FDG-PET revealed a reduction of glucose metabolism of the treated putamen relative to the contralateral putamen in all patients.Introduction
Magnetic resonance-guided focused ultrasound (MRgFUS), combined with intravascular microbubbles, is an emerging technology that can transiently enhance the permeability of the blood-brain barrier (BBB) and augment drug delivery to brain tissues1-4. In this phase I trial5, a 55 kDa recombinant form of the human beta-glucocerebrosidase enzyme was delivered biweekly by MRgFUS to the putamen, a key brain structure related to movement disorder in Parkinson’s disease (PD).Methods
The study was approved by our institutional Research Ethics Board. Four patients with PD were enrolled and each underwent three biweekly treatments, during which beta-glucocerebrosidase (Cerezyme; Genzyme, Cambridge, MA, USA) was delivered to the putamen unilaterally (dose at 15, 30 and 60 IU/kg for the 3 treatments, respectively) by a modified clinical MRgFUS system (ExAblate 4000, 220 kHz, InSightec, Tirat Carmel, Israel) with a 3T MR scanner (Magnetom Prisma, Siemens Healthineers, Erlangen, Germany). Intraoperative 2D T2-weighted images of three orthogonal planes (turbo spin-echo, TR: 6500 ms, TE: 98 ms, in-plane resolution: 0.9 mm x 0.9 mm, slice thickness: 2 mm) were acquired for targeting putamen structures. Microbubbles (Definity; Lantheus, North Billerica, MA, USA) were infused intravenously via a saline bag gravity drip at an infusion rate of 4 µL/kg per 5 min. Acoustic power was controlled automatically by a cavitation dose-based feedback controller. T2*-weighted images (gradient echo, TR: 444 ms, TE: 20 ms, in-plane resolution: 0.9 mm x 0.9 mm, slice thickness: 3 mm) were acquired following a series of initial sonications to assess whether the target cavitation dose levels were appropriate for avoiding red blood cell (RBC) extravasations. If suspected regions of signal hypointensity were detected in T2*-weighted MRI, cavitation dose levels were lowered for subsequent sonications. Following treatment, the stereotactic frame was removed and follow-up MR images were acquired with an 8-channel head coil for improved image quality. The MRI contrast agent Gadovist (1.0 mmol/ml; Bayer AG, Germany) was injected at a dose of 0.1ml/kg, and 3D T1-weighted gradient-echo images (T1-MP RAGE, TR: 2000 ms, TE: 3 ms, TI: 900 ms, isotropic spatial resolution: 0.9 mm) were acquired following a delay of approximately 15 minutes to detect BBB opening. T2*-weighted images were re-acquired to monitor for indications of RBC extravasations produced within the target volumes. The MRI protocol was repeated one day following each treatment session to confirm restoration of BBB integrity. Fluorodeoxyglucose positron emission tomography (FDG-PET) was acquired before and 1-month after treatments to exam changes of putaminal metabolism.Results
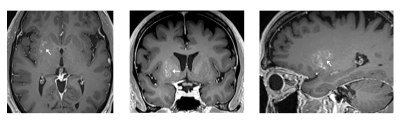
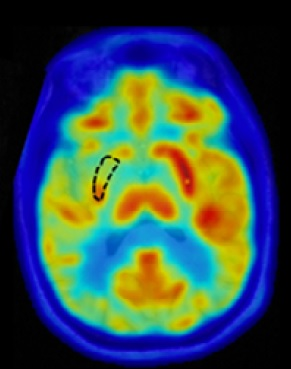
BBB permeability within the targeted putamen was elevated successfully in all treatments, as revealed by Gd-enhanced T1-weighted MRI immediately post treatment (Fig.1). The Gd-enhanced signal intensity within the targeted volume was increased by 14% ± 6% relative to the untreated contralateral side (mean ± standard deviation). No contrast enhancement was observed in the treated putamen on MR imaging scans acquired one day following each treatment session, indicating closure of the BBB. In one patient, post first-treatment T2*-weighted imaging revealed a small number of isolated hypointense spots within the treated volume, which were resolved the next day on follow-up imaging. Although it was not definitively clear whether these hypointense T2*-weighted signals were caused by RBC extravasations, the target cavitation dose level was reduced in the two subsequent treatments for this patient. FDG-PET revealed a reduction of glucose metabolism of the treated putamen relative to the contralateral putamen in all 4 patients (Fig.2).Discussion
Hypermetabolism in the putamen has been shown to correlate with PD progression and to be reduced following effective treatment. Therefore, the finding of metabolic reduction in the treated putamen by Cerezyme is encouraging and warrants further investigations. Technically, although acoustic power is adaptively controlled on the current MRgFUS system with the cavitation dose-based feedback controller, intraoperative and post-treatment T2*-weighted and Gd-enhanced T1-weighted MRI remain important for procedural feedback to adjust cavitation dose target levels for individual patients. While this trial successfully demonstrated that repeated BBB opening of the putamen can be performed safely, reversibly, and with good targeting accuracy and spatial coverage, further technical advancements are warranted to improve its performance for use across a wide variety of brain diseases.Acknowledgements
This study was supported by the Focused
Ultrasound Foundation and the Sunnybrook Foundation. The authors would like to thank
MRI technicians Ruby Endre and Garry Detzler for their support, and the
Anesthesia Department at Sunnybrook Health Sciences Centre for their services
related to patient management.
References
1. Hynynen K, McDannold N, Vykhodtseva N, et al. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 2001;220:640-6.
2. Mainprize T, Lipsman N, Huang Y, et al. Blood-brain barrier opening in primary brain tumors with non-invasive MR-guided focused ultrasound: a clinical safety and feasibility study. Sci Rep. 2019;9:321.
3. Lipsman N, Meng Y, Bethune AJ, et al. Blood-brain barrier opening in Alzheimer's disease using MR-guided focused ultrasound. Nat Commun. 2018;9:2336.
4. Pineda-Pardo JA, Gasca-Salas C, Fernández-Rodríguez B, et al. Striatal blood-brain barrier opening in Parkinson's disease dementia: a pilot exploratory study. Mov Disord. 2022;37:2057-65.
5. Meng Y, Pople CB, Huang Y, et al. Putaminal rGCase delivery with MR-guided focused ultrasound in Parkinson’s disease: a phase I study. Mov Disord. 2022;37:2134-9.