1385
Spatiotemporal Patterns of Iron Accumulation and Oxygen Metabolism in Patients with Parkinson's Disease1Department of Radiology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, 2MR Research, GE Healthcare, Beijing, China
Synopsis
Keywords: Parkinson's Disease, Quantitative Susceptibility mapping
The purpose of this study was to employ QSM and OEF maps to quantitatively measure iron content and oxygen metabolism levels in deep gray matter of the Parkinson’ s disease brains in different stages and explore their relationship with clinical features. Iron accumulation in SN, RN and GP significantly increased with the progression of PD severity. In contrast, OEF in CAU, PT, and DN elevated in early PD and then gradually decreased with worsening symptoms, indicating a different spatiotemporal pattern. These findings may facilitate to understand the neurobiological mechanisms in PD progression and offered complementary indicators for diagnosis and prediction.
Background or Purpose
Parkinson's disease (PD) is the second most common age-related neurological disorder. PD manifests clinical symptoms such as resting tremor, bradykinesia, rigidity, or postural instability1. Previous studies have offered evidence for neurodegeneration in the form of decreased cerebral glucose uptake and metabolism2, 3, and the abnormal accumulation of iron4, 5. However, the patterns of cerebral iron deposition and oxygen utilization in different stages of PD are still unknown. In this study, we aimed to explore alterations of quantitative susceptibility mapping (QSM) and oxygen extraction fraction (OEF) values in deep gray matter with PD progresses to explore spatiotemporal patterns in brain iron accumulation and oxygen metabolism. This may indicate potential clinicopathological lesions and inform targeted therapeutic strategies.Methods
With approval of the Institutional Review Board and signed informed consent of all subjects, eighty-seven PD patients with different stages and forty healthy controls were recruited in our study. All participants underwent MRI examinations including 3D T1-weighted brain volume imaging and multi-echo gradient echo (mGRE) sequence on a 3.0 T MR scanner (Discovery MR750, GE Health Care, Waukesha, WI, USA). The Hoehn & Yahr (H-Y) stage and the third part of the Unified Parkinson's Disease Rating Scale (UPDRS-III) were used to measure PD severity and motor symptoms. We further subdivided PD patients into early-stage PD (EPD) and late-stage PD (LPD) subgroups according to the H-Y stage. QSM maps were reconstructed from mGRE images using the morphology-enabled dipole inversion with automatic uniform cerebrospinal fluid zero reference algorithm (MEDI+0)6, and OEF maps were estimated by combined QSM modelling to process GRE phases and quantitative blood oxygen level–dependent (qBOLD) modeling to process GRE magnitudes7. By the age-specific QSM template and ANTs-SyN registration process, the subcortical nuclei in the basal ganglia, midbrain, and cerebellum were semi-automatically segmented, and the mean QSM and OEF values within each nucleus were extracted.Results
Iron deposition in most subcortical nuclei followed an ascending trend. After multiple comparisons correction, there were significant differences on the susceptibility values of the globus pallidum (GP), substantia nigra (SN) and red nucleus (RN) among the HCs, EPD and LPD groups (p < 0.001). The variation curve of cerebral oxygen metabolism in the gray matter nuclei showed an increasing and then decreasing trend. Mean OEF values of the caudate nucleus (CAU) and dentate nucleus (DN) were higher in EPD patients as compared with HCs, while LPD patients showed lower levels of oxygen metabolism in the structures including CAU, DN, putamen (PT), and thalamus relative to EPD patients. In addition, there were correlations between these observed abnormal QS and OEF values in the subcortical nuclei and clinical measures (H-Y stage, UPDRS-III scores). By combining the QSM values of SN and RN, and OEF values of CAU and DN, the AUC values can be improved to 0.836 for early PD diagnosis.Discussions and Conclusions
In this study, QSM and OEF maps were utilized to quantify stage-specific regional iron deposition and oxygen metabolism of deep grey nuclei structures in PD patients. Progressively increased iron content of SN, RN and GP was observed in PD, while the rising iron accumulation in GP was not significantly different to HCs until the late stage of PD. Continuously elevated iron deposition of these deep grey matter nuclei in PD has also been reported by previous studies8. The most prominent pathological hallmark of PD is the loss of dopaminergic neurons in substantia nigra of the midbrain, as well as synuclein-containing Lewy bodies9. Excessive iron in the brain could affect dopamine metabolism pathways, which produce neurotoxins and eventually lead to neuronal death10. This process may be accompanied by secondary degenerative changes in other regions of midbrain and the basal ganglia11. Furthermore, correlation analysis showed the QS values in SN, RN, PT and DN to be significantly correlated with H-Y stage and UPDRS-III scores. Iron deposition in both SN and other gray matter nuclei could monitor disease progression.In addition to iron deposition, mitochondrial dysfunction and abnormal cerebral oxygen metabolism are also important features of PD pathology. Brain fluorodeoxyglucose positron emission tomography (FDG-PET) imaging studies have identified a PD-related pattern characterized by relative hypermetabolism in thalamus, pallidum, putamen, pontine, and cerebellum, and hypometabolism in posterior parietal, occipital, and frontal cortices3, 12. In our study, we found that EPD patients had higher mean OEF values in CAU, PUT and DN as compared to those of HCs and LPD patients. Therefore, we speculated that the enhanced oxygen metabolism in early PD may be attributed to oxidative stress conditions as well as inflammatory responses in microglia; the subsequent decrease in oxygen extraction fraction might reflect neuronal loss.
Regional and temporal progression patterns of brain iron deposition and oxygen metabolism are distinct in Parkinson's disease. This may reflect different pathological alterations in the neurodegenerative process, which could provide a better understanding of the neurobiology as complementary information and offer indicators for early diagnosis and monitoring of the disease.
Acknowledgements
This project was supported by the National Natural Science Funds of China (Grants No.81730049).References
1. Maiti P, Manna J, Dunbar GL. Current understanding of the molecular mechanisms in Parkinson's disease: Targets for potential treatments. Transl Neurodegener. 2017;6:28. doi:10.1186/s40035-017-0099-z
2. Albrecht F, Ballarini T, Neumann J, Schroeter ML. FDG-PET hypometabolism is more sensitive than MRI atrophy in Parkinson's disease: A whole-brain multimodal imaging meta-analysis. Neuroimage Clin. 2019;21:101594. doi:10.1016/j.nicl.2018.11.004
3. Meles SK, Renken RJ, Pagani M, et al. Abnormal pattern of brain glucose metabolism in Parkinson's disease: replication in three European cohorts. Eur J Nucl Med Mol Imag. Feb 2020;47(2):437-450. doi:10.1007/s00259-019-04570-7
4. Wang JY, Zhuang QQ, Zhu LB, et al. Meta-analysis of brain iron levels of Parkinson's disease patients determined by postmortem and MRI measurements. Sci Rep. Nov 9 2016;6:36669. doi:10.1038/srep36669
5. Guan X, Xu X, Zhang M. Region-Specific Iron Measured by MRI as a Biomarker for Parkinson's Disease. Neurosci Bull. Oct 2017;33(5):561-567. doi:10.1007/s12264-017-0138-x
6. Liu Z, Spincemaille P, Yao Y, Zhang Y, Wang Y. MEDI+0: Morphology enabled dipole inversion with automatic uniform cerebrospinal fluid zero reference for quantitative susceptibility mapping. Magn Reson Med. May 2018;79(5):2795-2803. doi:10.1002/mrm.26946
7. Cho J, Kee Y, Spincemaille P, et al. Cerebral metabolic rate of oxygen (CMRO(2) ) mapping by combining quantitative susceptibility mapping (QSM) and quantitative blood oxygenation level-dependent imaging (qBOLD). Magn Reson Med. Oct 2018;80(4):1595-1604. doi:10.1002/mrm.27135
8. He N, Ling H, Ding B, et al. Region-specific disturbed iron distribution in early idiopathic Parkinson's disease measured by quantitative susceptibility mapping. Hum Brain Mapp. Nov 2015;36(11):4407-20. doi:10.1002/hbm.22928
9. Braak H, Tredici KD, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003/03/01/ 2003;24(2):197-211. doi:https://doi.org/10.1016/S0197-4580(02)00065-9
10. McCann H, Cartwright H, Halliday GM. Neuropathology of α-synuclein propagation and braak hypothesis. Mov Disord. Feb 2016;31(2):152-60. doi:10.1002/mds.26421
11. Kordower JH, Olanow CW, Dodiya HB, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain. Aug 2013;136(Pt 8):2419-31. doi:10.1093/brain/awt192
12. Wu P, Wang J, Peng S, et al. Metabolic brain network in the Chinese patients with Parkinson's disease based on 18F-FDG PET imaging. Parkinsonism Relat Disord. Jun 2013;19(6):622-7. doi:10.1016/j.parkreldis.2013.02.013
Figures
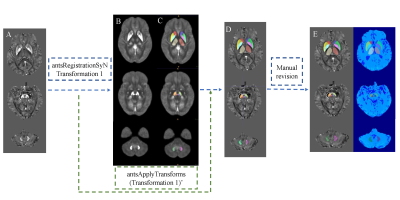
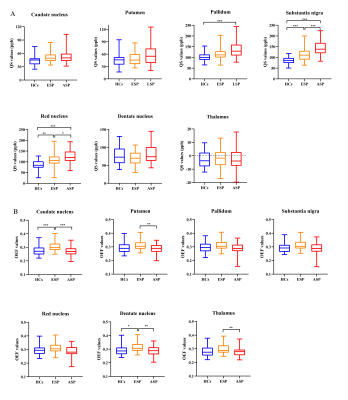
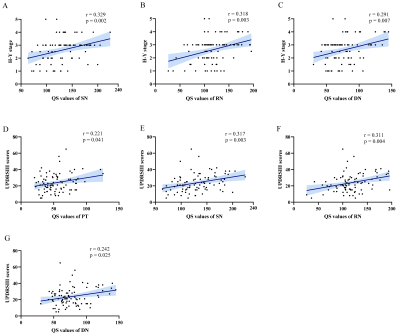
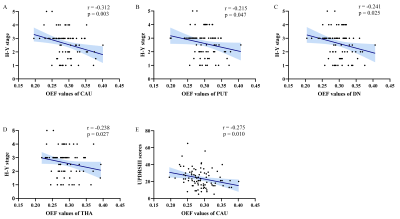
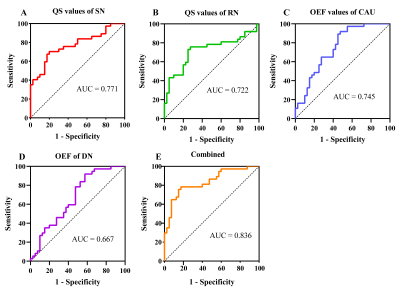
Figure 5 Receiver operating characteristic (ROC) analysis of the mean QSM and OEF values in subcortical nuclei using logistic regression to identify early-stage Parkinson's disease patients. The diagnostic performance was defined by the area under the curve (AUC). QSM, quantitative susceptibility mapping; OEF, oxygen extraction fraction; SN, substantia nigra; RN, red nucleus; CAU, caudate nucleus; DN, dentate nucleus.