1383
Progressive cortical cerebrovascular reactivity reduction occurs in Parkinson's disease: a longitudinal study
Hongwei Li1, Jian Wang2,3, Jia Jia4, Xiali Shao2, He Wang1,5,6, and Lirong Jin4
1Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China, 2Department of Radiology, Zhongshan Hospital, Fudan University, Shanghai, China, 3Department of Radiology, Zhongshan Hospital, Fudan University (Xiamen Branch), Xiamen, China, 4Department of Neurology, Zhongshan Hospital, Fudan Universit, Shanghai, China, 5Human Phenome Institute, Fudan University, Shanghai, China, 6Key Laboratory of Computational Neuroscience and Brain-Inspired Intelligence (Fudan University), Ministry of Education, Shanghai, China
1Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China, 2Department of Radiology, Zhongshan Hospital, Fudan University, Shanghai, China, 3Department of Radiology, Zhongshan Hospital, Fudan University (Xiamen Branch), Xiamen, China, 4Department of Neurology, Zhongshan Hospital, Fudan Universit, Shanghai, China, 5Human Phenome Institute, Fudan University, Shanghai, China, 6Key Laboratory of Computational Neuroscience and Brain-Inspired Intelligence (Fudan University), Ministry of Education, Shanghai, China
Synopsis
Keywords: Parkinson's Disease, fMRI (resting state), cerebrovascular reactivity
Increasing evidence showed subtle cerebrovascular reactivity (CVR) impairment in neurodegenerative disease. In the longitudinal study herein, we aimed to investigate regional CVR changes in the patients with Parkinson’s disease (PD) at baseline and 2 years follow up, and CVR was derived from resting state fmri scans. PD patients showed significantly reduced CVR in the left inferior occipital gyrus and right superior temporal cortex. In addition, the reduction of CVR may associate with executive function deficits. Our results also showed that there was a tendency for functional connectivity to be weakened from posterior to anterior with the progression of the disease.Introduction
Parkinson’s disease (PD) is a common progressive neurodegenerative disease. Apart from dopaminergic neurons loss in the substantia nigra, vascular alternation might be a contributor to the disease progression1. It is worthy to elicit that neurovascular unit (NVU) disorders have been reported to be involved in the mechanism of PD2,3. However, its longitudinal alteration in PD progression in vivo remains unclear. Cerebrovascular reactivity mapping without gas challenges, is a noninvasive and feasible method to detect the NVU alteration, especially suitable for the repeated examination in PD patients. In this study, we aimed to investigate regional CVR changes in the patients with PD at baseline and 2-years follow up. Furthermore, cognitive impairment is a common non-motor symptom and potentially occur at any disease stage in PD, while the reasons remain unclear. Since lower CVR has been reported to predict cognitive decline in normal adults4, the second goal of this study was to examine whether longitudinal CVR alternation are associated with cognitive impairment. Finally, we wanted to detect brain functional connectivity alternation in PD patients over time, secondary to microvascular changes.Materials and methods
Subjects and clinical assessmentsWe recruited 90 PD patients and 51 matched healthy control subjects (HCs). PD patients completed a comprehensive battery of neuropsychological assessments and clinical evaluations at baseline (V0) and two-year follow-up visit (V1). HCs completed neuropsychological assessments at baseline.
Image acquisition
All MR data were acquired using a 3-Tesla MR unit (Discovery™ MR750, GE Healthcare, Milwaukee, WI). BOLD images were acquired by using single-shot GRE-EPI. The parameters of BOLD sequence were: TR = 2000ms, TE = 30ms, flip angle = 90°, FOV = 24cm, matrix = 64×64, number of slices = 34, slice thickness = 4.0 mm, number of dynamic scans = 210. High-resolution 3D BRAVO T1-weighted images: TR = 8.2ms, TE = 3.2ms, TI = 450ms, flip angle = 12°, FOV = 24cm, matrix = 256×256.
Image processing
For resting-state BOLD images, after motion correction, smoothing, and linear detrending, the rs-BOLD data were temporally filtered with a band-pass filter of 0.01 to 0.1Hz. And then the voxel-wise regression analysis was performed to generate a CVR index map5, in which the whole brain signal was treated as an independent variable in this general linear model. For functional connectivity analysis, the seeds were selected from CVR paired t-tests and linear regression results, and the FC was measured by a Pearson correlation between the seeds and each voxel time courses. The Fisher-transformed z-score of FC maps were used for subsequent statistical analysis. For structural images, cortical thickness was extracted using the CAT12 toolbox pipeline for surface-based morphometry6.
Results
In group comparisons, a voxel-wise two-sample t-test was performed between control and patient groups, and a voxel-wise paired t-test was used to detect longitudinal changes over two years in PD patients. As shown in Figure 1, compared with HCs, PD patients showed significantly reduced CVR in the left inferior occipital and right superior temporal cortex at V0. At V1, the cluster constricted to the same location was larger and more significant. More importantly, PD patients showed a significant CVR reduction in the left inferior occipital cortex over time. Furthermore, correlations between longitudinal changes in CVR and clinical assessments were measured. From V0 to V1, after FDR correction, multiple linear regression analysis showed that longitudinal left posterior cingulate cortex (PCC) CVR reduction was negatively associated with decline of performance on the Trail Making Test B (TMT-B) in PD patients, controlling for decreased cortical thickness, changes of UPDRS motor scores, changes of Geriatric Depression Rating Scales scores, interval of two visits, years of education and gender (Figure 2). On the other hand, comparison between HCs and the PD patients at V0 showed weakened FC between left inferior occipital cortex and posterior regions such as cerebellum, temporal, occipital and parietal cortex, while the PD patients at V1 exhibited widespread weaker left inferior occipital cortex FC with the posterior-anterior region, especially with frontal cortex, including middle, medial, superior and inferior frontal cortex (Figure 3A-D). In addition, the left PCC in HCs was more strongly connected with bilateral superior occipital, bilateral middle occipital, left inferior occipital gyrus and left precuneus than the PD patients at V0 (Figure 4A-B). Except for occipital cortex, the left PCC in HCs were more strongly connected with cuneus and bilateral fusiform gurus in respect to PD patients at V1(Figure 4C).Discussion and Conclusion
In this study, we found the longitudinal changes in CVR over two years in PD patients in comparison with controls, suggesting that microvascular dysfunction might be involved in disease progression. In addition, the results presented that longitudinal CVR reduction in left PCC was negatively correlated with increased completion time of TMT-B. This finding implied progressive decreased CVR might partly contribute to executive function deficits. Even more, our preliminary results showed functional connectivity was weakened following posterior-anterior distribution. This finding also implicated dysfunction of NVU, secondary to or synchronize brain function as the disease progressed. To our knowledge, the present study first demonstrated spatial and temporal related CVR changes and their association with cognitive impairment in PD patients. Our results contribute to a better standing of vascular role in PD progression, especially in cognitive decline.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81971583), National Key R&D Program of China (No. 2018YFC1312900), Shanghai Natural Science Foundation (No. 20ZR1406400), Shanghai Municipal Science and Technology Major Project (No.2017SHZDZX01, No.2018SHZDZX01) and ZJLab, Young and Middle-aged Health Talents Training Project of Fujian Province (No. 2020GGB059).References
1. Paul, G. & Elabi, O.F. Microvascular Changes in Parkinson’s Disease- Focus on the Neurovascular Unit. Frontiers in Aging Neuroscience 14(2022).
2. Chen, X., Lan, X., Roche, I., Liu, R. & Geiger, J.D. Caffeine protects against MPTP-induced blood-brain barrier dysfunction in mouse striatum. Journal of Neurochemistry 107, 1147-1157 (2008).
3. Banks, W.A., et al. Lipopolysaccharide-induced blood-brain barrier disruption: roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. Journal of Neuroinflammation 12, 223 (2015).
4. Peng, S.-L., et al. Age-related changes in cerebrovascular reactivity and their relationship to cognition: A four-year longitudinal study. NeuroImage 174, 257-262 (2018).
5. Liu, P., et al. Cerebrovascular reactivity mapping without gas challenges. NeuroImage 146, 320-326 (2017).
6. Dahnke, R., Yotter, R.A. & Gaser, C. Cortical thickness and central surface estimation. NeuroImage 65, 336-348 (2013).
Figures
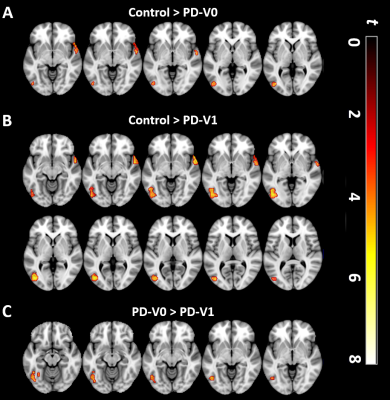
Figure 1. Comparisons of regional CVR among the PD group at baseline (V0), PD group at follow up (V1), and healthy controls. (A) At V0, PD patients showed significant CVR reduction in the left inferior occipital gyrus and right temporal cortex, compared with controls. (B) At V1, PD patients showed a significant larger CVR reduction in the same region than controls. (C) Left inferior occipital CVR was significantly decreased between PD patients at V0 and V1 over time. SPM t-maps are overlaid on a T1 MNI template. All results were thresholded at p-FDR <0.05 cluster level.
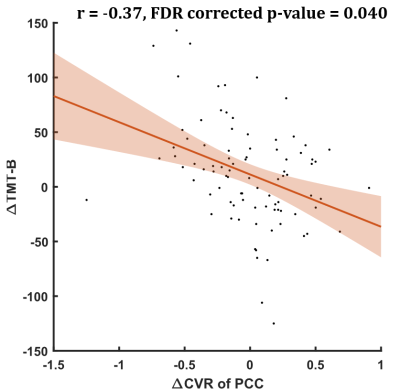
Figure 2. The prolonged completion time of Trail Making Test-B (TMT-B) was negatively correlated with longitudinal CVR reduction in the left posterior cingulate cortex.
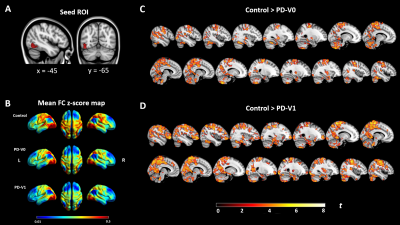
Figure 3. Functional connectivity comparison between PD patients and Controls. (A) Seed ROI (left inferior occipital gyrus, IOG.L) was selected from cluster of CVR paired t-tests, as shown in Figure 1. (B) Seed-to-voxel FC z-score maps of healthy controls and PD at baseline and 2 years follow-up visit. (C) Significant FC reduction between IOG.L and posterior regions in PD at V0, compared with HCs. (D) Significant FC reduction between IOG.L and posterior-anterior regions in PD at V1 in contrast with HCs. All results were thresholded at p-FDR <0.05 cluster level.
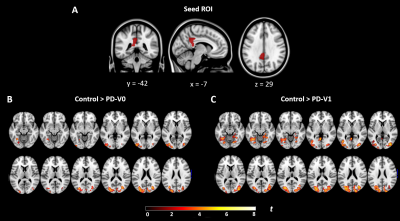
Figure 4. Changes of functional connectivity in patients with PD over time, compared to controls. (A) Seed ROI (left posterior cingulate cortex) was selected from cognitive decline related regional CVR. (B) The left PCC in PD patients at V0 were significantly weaker connection with occipital cortex and left precuneus than the controls. (C) The left PCC in PD patients at V1 were significantly weaker connection with occipital cortex, cuneus gyrus and bilateral fusiform gyrus in comparison with the controls. All results were thresholded at p-FDR <0.05 cluster level.
DOI: https://doi.org/10.58530/2023/1383