1367
Suppressing blood signal in myocardial T1ρ mapping at 3T through novel dark-blood adiabatic spin-lock preparations.1TU Delft, Delft, Netherlands, 2Heidelberg University, Heidelberg, Germany, 3HollandPTC, Delft, Netherlands, 4University of Minnesota, Minneapolis, MN, United States
Synopsis
Keywords: Relaxometry, New Signal Preparation Schemes, T1ρ, Dark-blood, adiabatic
T1ρ-mapping is emerging as a promising, contrast-free alternative to LGE for assessment of myocardial viability. However, high blood T1ρ values can obfuscate endocardial scar. In this work, we propose a dark-blood adiabatic T1ρ preparation to suppress the blood signal and improve depiction of the blood-myocardium interface. A slice-selection gradient is added to an odd number of adiabatic full passage pulses to achieve blood inversion outside the imaging slab. Phantom results show that DBT1ρ yields unbiased estimation of T1ρ time. In vivo, thorough blood suppression is achieved for the trade-off against a moderate increase in DBT1ρ variance.Introduction
Late gadolinium enhancement (LGE) remains the clinical gold standard for the assessment of myocardial viability. In LGE, blood appears as bright as the myocardium, thus the detection of subendocardial lesions can be challenging. To alleviate this, dark-blood (DB) LGE has been recently proposed, nulling the healthy myocardium and attenuating the blood signal[1]. T1ρ mapping is an emerging non-contrast alternative to LGE for the discrimination of healthy and infarcted myocardium, but faces similar problems at the sub-endocardium [2-5]. The blood pool is characterized by high T1ρ, potentially obfuscating endocardial scar. In this study, we propose a novel adiabatic T1ρ preparation to achieve dark-blood contrast in T1ρ-adiab mapping for improved depiction of the blood-myocardial interface.Methods
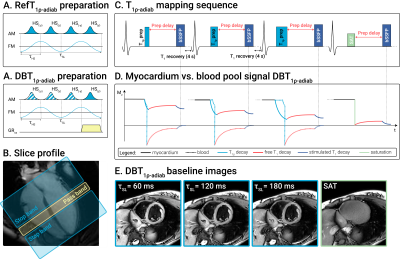
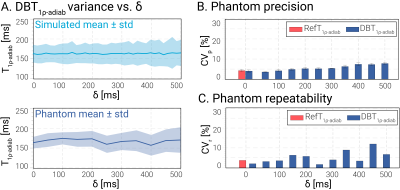
Previously proposed bright-blood adiabatic spin-lock (SL) preparations were used to acquire reference T1ρ-adiab maps (RefT1ρ-adiab)[6]. These consisted of trains of even-numbered hyperbolic secant (HS) adiabatic full passage (AFP) pulses (τHS=30ms, β=6.9, fmax=450Hz)(Fig 1a). DB contrast was achieved by playing a 880μT/m slice-selection gradient for all HS pulses in the preparation train, except for the last. This way, an even number of AFPs is applied to the labeling slab, inducing T1ρ-adiab decay in the imaging slice. Outside the slab, only the last AFP is experienced, leading to magnetization inversion (Fig 1b). A delay (δ) was added between the preparation and the acquisition to allow for the inverted blood to flow into the imaging slab. The preparation duration was maximized for each volunteer, depending on the subject’s heart rate (δ=320-750ms). Bloch simulations and phantom imaging with δ=0-500ms were performed to assess the influence of the delay on the T1ρ-adiab variance. T1ρ-adiab mapping was performed on a 3T scanner (Philips Ingenia) with a 14s breath-hold cardiac-triggered snap-shot bSSFP sequence (Fig 1c). Three T1ρ-prepared images (𝜏SL=60, 120, 180ms for 2, 4, HS pulses) were acquired, interleaved by 4s Mz recovery pauses and followed by a saturation-prepared image[6]. Other sequence parameters were: resolution=2x2x8mm3, FOV=220x220mm2, TE/TR=0.76/1.90ms. All T1ρ-adiab maps were generated using a three-parameter model[7]. Precision (CVp) and repeatability (CVr) were assessed in the T1MES phantom[8] for RefT1ρ-adiab and DBT1ρ-adiab with varying δ. Ten repetitions were acquired for each case. Finally, the sequence was tested in four healthy volunteers (1 female, 3 males, 27±5y/o). For each volunteer, 3 repetitions of mid short-axis (SAX) were acquired to assess repeatability, in addition to single apical SAX, basal SAX and 4 chambers views. CVp, CVr and inter-subject variability (CVs), were investigated in manually segmented myocardium ROIs.Results
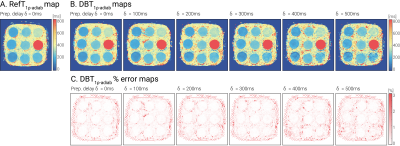
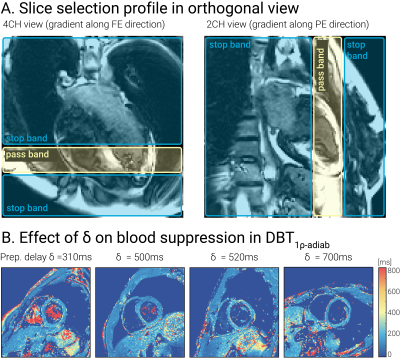
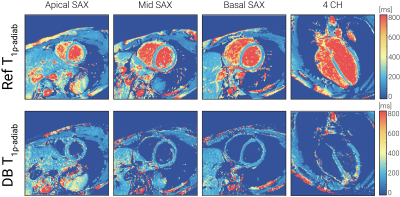
T1ρ-adiab maps obtained with RefT1ρ-adiab and DBT1ρ-adiab show good agreement for all preparation delays (Fig 2, myocardium-like vial: RefT1ρ-adiab: 184.73±7.83ms, DBT1ρ-adiab with δ=500ms: 188.68±18.73ms). Precision and repeatability were comparable to RefT1ρ-adiab when no preparation delay was used (Fig 3b-c, DBT1ρ-adiab with δ=0ms: CVp=4.24±0.49%, CVr=3.72% vs RefT1ρ-adiab: CVp=4.00±0.56%, CVr=2.03%). However, larger variability is observed for increasing δ, due to magnetization relaxation after the preparation (Fig 3a, linear regression: slope=19.61, intercept=7.38, R2=0.81, p-value<<0.05). In-vivo testing of the preparation with slice-selection gradient along the phase and frequency-encoding dimensions, shows sharp delineation of an inversion and a pass band (Fig 4a). When being played along the slice-selection direction, thorough blood suppression is observed (Fig 4b). For subjects with high heart rates, where only short δ was possible, complete suppression of the blood is compromised (Fig 4b). However, the blood near the myocardial interface remains thoroughly nulled. In vivo, homogenous and artifact-free T1ρ-adiab quantification is observed with both RefT1ρ-adiab and DBT1ρ-adiab (Fig. 5). No significant difference was found between myocardial DBT1ρ-adiab values and the reference (DBT1ρ-adiab=197.15±42.31ms, RefT1ρ-adiab=202.03±26.45ms, p>0.05). DBT1ρ-adiab maps show compromised precision, repeatability and inter-subject variability (DBT1ρ-adiab CVp=24.62±5.10%, CVr=12.23±3.84%, CVs=14.67%, vs RefT1ρ-adiab CVp=12.66±2.09%, CVr=7.16±1.90%, CVs=11.28%, p<0.05).Discussion
In this study we explored the use of dark-blood contrast in adiabatic T1ρ mapping in the myocardium. Our results show that DBT1ρ-adiab maps yield quantification and map quality comparable to conventional RefT1ρ-adiab, while achieving thorough blood signal suppression. Increasing preparation delays resulted in improved blood suppression by allowing nulling of the inverted blood magnetization and its inflow to the pass band, albeit at the trade-off against reduced precision. In the presence of short preparation delays, increased imaging flip angles could be used to drive the blood signal closer to the zero crossing, for the trade-off against decreased contrast sensitivity. The proposed implementation includes the acquisition of a saturation-prepared image with equal delay. This allows to capture both the magnetization relaxation, as well as the effect of the imaging readout. In combination with a 3 parameter fit this enables unbiased DBT1ρ-adiab estimation, irrespective of the preparation delay.Conclusions
DBT1ρ-adiab preparations enable thorough blood signal suppression in adiabatic T1ρ mapping, at a moderate increase in T1ρ-adiab quantification variance. Thus, DBT1ρ-adiab forms a promising alternative to conventional T1ρ mapping with the potential to enhance visualization of subendocardial scar.Acknowledgements
Funding:4TU Precision Medicine program, NWO Start-up STU.019.024, ZonMW OffRoad grant 04510011910073.References
[1]Basha, T.A., Tang, M.C., Tsao, C., et al., Improved dark blood late gadolinium enhancement (DB-LGE) imaging using an optimized joint inversion preparation and T2 magnetization preparation. Magn. Reson. Med., 79: 351-360 (2018).
[2] Witschey, W.R., Zsido, G.A., Koomalsingh, K. et al. In vivo chronic myocardial infarction characterization by spin locked cardiovascular magnetic resonance. J Cardiovasc Magn Reson 14, 37 (2012)
[3] van Oorschot, J.W., El Aidi, H., Jansen of Lorkeers, S.J. et al. Endogenous assessment of chronic myocardial infarction with T1ρ-mapping in patients. J Cardiovasc Magn Reson 16, 104 (2014).
[4] Stoffers, R. H., Madden, M., Shahid, M., Contijoch, F., Solomon, J., Pilla, J. J., ... & Witschey, W. R. (2017). Assessment of myocardial injury after reperfused infarction by T1ρ cardiovascular magnetic resonance. Journal of cardiovascular magnetic resonance, 19(1), 1-10.
[5] Bustin, A., Toupin, S., Sridi, S. et al. Endogenous assessment of myocardial injury with single-shot model-based non-rigid motion-corrected T1 rho mapping. J Cardiovasc Magn Reson 23, 119 (2021).
[6] Coletti, C. et al. Adiabatic T1ρ mapping of the human myocardium at 3T. ISMRM 2022:0273.
[7] Akçakaya M, Basha TA, Weingärtner S, et al. Improved quantitative myocardial T2 mapping: Impact of the fitting model. Magn Reson Med. (2015).
[8] Captur, G., Bhandari, A., Brühl, R. et al. T1 mapping performance and measurement repeatability: results from the multi-national T1 mapping standardization phantom program (T1MES). J Cardiovasc Magn Reson 22, 31 (2020)
Figures