1365
Elevated Metabolic-functional Coupling in Epileptogenic Lesion and Network are Related with Surgical Outcomes of Mesial Temporal Lobe Epilepsy1School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China, 2Department of Nuclear Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, 3Department of Neurosurgery, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Synopsis
Keywords: fMRI, PET/MR
It has been unclear whether metabolic-functional coupling could be disturbed in epileptogenic lesion and network by epileptic activities. In this study, we investigated metabolic changes, functional alterations and their couplings in epileptogenic lesion and network of mesial temporal lobe epilepsy (MTLE) using simultaneous PET/MR, and further evaluated the relationship between altered couplings and surgical outcome. MTLE patients showed abnormalities of standardized uptake value ratio (SUVR) and fractional amplitude of low-frequency fluctuation (fALFF) in default mode network (DMN). MTLE patients with hippocampal sclerosis (MR-HS) had higher SUVR-fALFF coupling in ipsilateral hippocampus and DMN, and the couplings were associated with surgical outcomes.
Introduction:
Mesial temporal lobe epilepsy (MTLE) is the most common type of drug-resistant epilepsy, with 40% chance of surgical failure 2 years after effective surgical strategy1. Hippocampal sclerosis (HS) is the most common pathology underlying MTLE2. Emerging evidence indicated that MTLE was not merely a focal epilepsy of the hippocampus, but a network-based disease with widespread abnormalities throughout epileptogenic networks, including default mode network (DMN)3-7. A previous study in healthy subjects have found strong couplings between glucose metabolism and functional activity across the brain8. Although altered glucose metabolism and resting-state functional activity have been widely reported in MTLE3, 9, 10, it remains unclear whether the repeated seizures could disturb metabolic-functional coupling in MTLE. In this study, we investigated the changes in glucose uptake, functional activity, and their couplings in epileptogenic lesion and network of MTLE using simultaneous PET/MR, and further evaluated whether the couplings could predict surgical outcomes.Method:
Data acquisition:
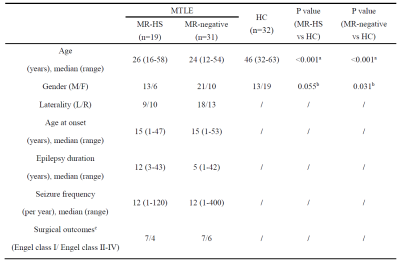
In this IRB-approved study, we recruited 50 drug-refractory unilateral MTLE patients between February 2019 and November 2021, and 32 healthy controls (HC). All subjects were scanned on a PET/MR scanner (Biograph mMR; Siemens Healthcare, Erlangen, Germany). The PET images were obtained at 30~50 minutes following a bolus injection of 3.7 MBq/kg of 18F-FDG (voxel size = 2.0 × 2.0 × 2.0 mm3, matrix size = 344 × 344, 127 slices). The MR scan protocols included T1-weighted MPRAGE (1.0 × 1.0 × 1.0 mm3, FOV = 256 × 256 mm2, 192 slices, TR/TE = 1900/2.44 ms), T2-weighted FLAIR (0.4 × 0.4 × 3.0 mm3, FOV = 220 × 220 mm2, 45 slices, TR/TE = 8460/92 ms), and resting-state fMRI (3.0 × 3.0 × 3.0 mm3, TR/TE = 3000/30 ms, 200 time points). Other clinical records such as semiology, long-term video-EEG monitoring, radiological diagnosis of MR-HS and MR-negative cases, surgical type, and outcomes were collected. Twenty-four of all patients had 2 years follow-up after anterior temporal lobotomy (ATL) surgery, consisting of 14 seizure-free (Engel class Ⅰ) and 10 non-seizure-free (Engel class Ⅱ-Ⅳ) evaluated by the Engel Epilepsy Surgery Outcome Scale11.Data processing:
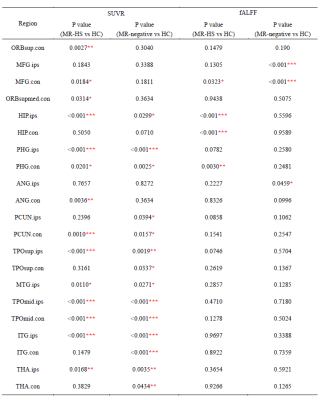
The individual cerebellar masks were extracted from the T1-MPRAGE using FreeSurfer image analysis v7.0 package. Standardized uptake value ratio (SUVR), quantifying the glucose metabolism, were obtained using intensity normalization by cerebellar scaling of 18F-FDG PET images, and then registered to MNI space. Resting state fMRI images were preprocessed using DPARSFA toolbox12, obtaining maps of fractional amplitude of low-frequency fluctuation (fALFF) in the bandpass 0.01 to 0.08 Hz13 in MNI space. Region-of-interest (ROI) based SUVR and fALFF were extracted for brain structures based on the mask of DMN14, and other masks from automated anatomical labelling (AAL) atlas15.Statistical analysis:
All the group comparation were performed using Mann-Whitney U tests. Chi-square was used to check gender difference between MTLE patients and healthy controls. We obtained the brain regions with significant changes in SUVR and fALFF in MR-HS and MR-negative group, compared to HC group. The spatial voxel-wise coupling between SUVR and fALFF in these brain regions were calculated using partial spearman correlation coefficient in each subject, considering grey matter fraction as covariate; then their differences among MR-HS, MR-negative and HC groups were evaluated. We further investigated whether the altered couplings in seizure-free group could differ from non-seizure-free group, and used logistic regression to evaluate their value of surgical outcomes prediction.Results and discussions:
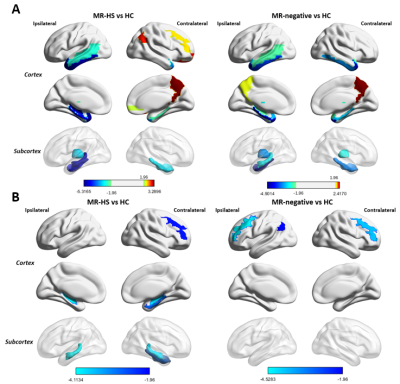
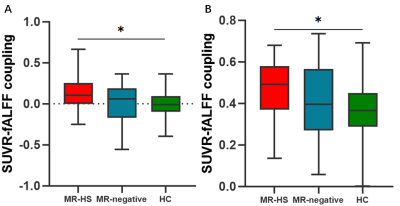
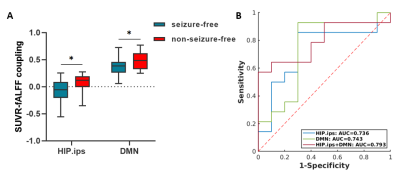
Detailed demographics and clinical characteristics of all participants were listed in Table 1. Healthy controls had higher ages than MR-HS and MR-negative patients, and the gender ratio was not matched between MR-negative group and healthy control group (Table 1). Thus, the group comparisons were performed after controlling for age and gender.MR-HS and MR-negative both had significantly reduced SUVR in regions of temporal lobe, such as hippocampus, parahippocampal gyrus, middle temporal pole, etc, while increased SUVR was found in several extratemporal regions forming DMN, compared to HC (Figure 1A, Table 2). Among DMN regions, ipsilateral hippocampus, contralateral parahippocampal gyrus and contralateral middle frontal gyrus were found to have lower fALFF in MR-HS group (Figure 1B, Table 2), while at the same time showing alterations in SUVR (Figure 1A, Table 2). Regarding metabolic-functional coupling, MR-HS showed positive SUVR-fALFF coupling in ipsilateral hippocampus where no coupling existed in HC hippocampus (Figure 2A). On the other hand, HC, MR-HS and MR-negative groups all had significant partial spearman correlation between SUVR and fALFF in DMN, while higher spatial voxel-wise coupling in MR-HS groups were found (Figure 2B). Furthermore, non-seizure-free group showed elevated coupling in DMN and ipsilateral hippocampus, compared with seizure-free group (Figure 3A). The surgical outcomes prediction performance of coupling in ipsilateral hippocampus and DMN were area under curve (AUC) of 0.736 and 0.743 respectively, and the combination of them resulted in AUC of 0.793 (Figure 3B).
Conclusion:
In this study, we found that MR-HS and MR-negative both showed hypometabolism in regions of temporal lobe, hypermetabolism in extratemporal regions forming DMN, and hypoactivity in DMN. Our findings also suggested that altered SUVR-fALFF coupling in ipsilateral hippocampus and DMN could improve the comprehending of the pathogenesis in MR-HS patients, and provide a potential neuroimaging metric for lateralization and surgical outcomes prediction in preoperative planning.Acknowledgements
N/AReferences
1. Liu JT, Liu B, Zhang H. Surgical versus medical treatment of drug-resistant epilepsy: A systematic review and meta-analysis. Epilepsy Behav. 2018;82:179-88.
2. Blumcke I. Neuropathology of focal epilepsies: a critical review. Epilepsy Behav. 2009;15(1):34-9.
3. Chassoux F, Artiges E, Semah F, Desarnaud S, Laurent A, Landre E, et al. Determinants of brain metabolism changes in mesial temporal lobe epilepsy. Epilepsia. 2016;57(6):907-19.
4. Tsuda K, Tsuji T, Ishida T, Takahashi S, Yamada S, Ohoshi Y, et al. Widespread abnormalities in white matter integrity and their relationship with duration of illness in temporal lobe epilepsy. Epilepsia Open. 2018;3(2):247-54.
5. Englot DJ, D'Haese PF, Konrad PE, Jacobs ML, Gore JC, Abou-Khalil BW, et al. Functional connectivity disturbances of the ascending reticular activating system in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2017;88(11):925-32.
6. Zanao TA, Lopes TM, de Campos BM, Yasuda CL, Cendes F. Patterns of default mode network in temporal lobe epilepsy with and without hippocampal sclerosis. Epilepsy Behav. 2021;121(Pt B):106523. 7. Pittau F, Grova C, Moeller F, Dubeau F, Gotman J. Patterns of altered functional connectivity in mesial temporal lobe epilepsy. Epilepsia. 2012;53(6):1013-23.
8. Aiello M, Salvatore E, Cachia A, Pappata S, Cavaliere C, Prinster A, et al. Relationship between simultaneously acquired resting-state regional cerebral glucose metabolism and functional MRI: a PET/MR hybrid scanner study. Neuroimage. 2015;113:111-21
9. Reyes A, Thesen T, Wang X, Hahn D, Yoo D, Kuzniecky R, et al. Resting-state functional MRI distinguishes temporal lobe epilepsy subtypes. Epilepsia. 2016;57(9):1475-84.
10. Wang J, Shan Y, Dai J, Cui B, Shang K, Yang H, et al. Altered coupling between resting-state glucose metabolism and functional activity in epilepsy. Ann Clin Transl Neurol. 2020;7(10):1831-42.
11. Engel J, Jr. Update on surgical treatment of the epilepsies. Summary of the Second International Palm Desert Conference on the Surgical Treatment of the Epilepsies (1992). Neurology. 1993;43(8):1612-7.
12. Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB Toolbox for "Pipeline" Data Analysis of Resting-State fMRI. Front Syst Neurosci. 2010;4:13.
13. Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 2008;172(1):137-41.
14. Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125-65.
15. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273-89.
Figures