1362
Simultaneous T1 and T2 relaxometry of the human brain at 7T using Quantitative Transient-state Imaging1IRCCS Stella Maris, Pisa, Italy, 2GE Healthcare, Munich, Germany, 3IMAGO7 Foundation, Pisa, Italy, 4Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy
Synopsis
Keywords: High-Field MRI, High-Field MRI
Fast quantitative MR methods enable repeatable and reproducible assessment of tissue properties improving diagnosis and follow-up. Here, we implemented two different 3D relaxometry methods based on quantitative transient-state imaging (QTI) for fast T1 and T2 mapping of the human brain at 7T. The two techniques were demonstrated both in-vitro and in-vivo and provided good quality parametric maps with low geometric distortion and blurring in clinically feasible acquisition and reconstruction times.Introduction
Quantitative MR relaxometry based on transient-state of MR signal, such as MR Fingerprinting1, MR-STAT2 and Quantitative Transient-state Imaging (QTI)3 has been shown promising in providing reproducible and repeatable assessment of biological tissues in a short acquisition time4–6. Despite the gain in terms of SNR and image resolution obtained with increasing field strength, most studies have been focused on 1.5T and 3T scanners because of the increased geometrical distortion and blurring at Ultra High Field (UHF) strengths (≥7T) and the bias induced in quantitative maps due to higher inhomogeneities of the transmit B1 field. Here, we designed two different 3D acquisition schemes (spiral and radial) for fast whole-brain simultaneous T1 and T2 mapping on a 7T scanner, demonstrating the feasibility of our techniques both in-vitro and in-vivo.Methods
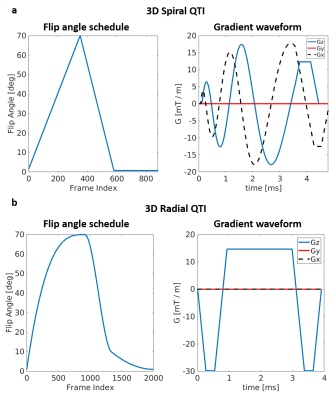
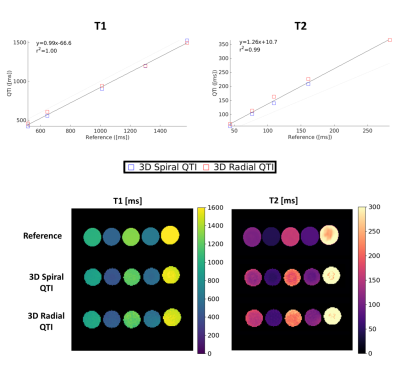
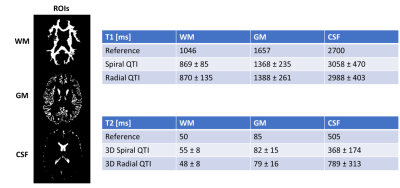
Acquisition design: We compared two different inversion-prepared 3D QTI variants, using spiral projections (3D Spiral QTI) and golden-means rotated radial spokes7 (3D Radial QTI) for k-space sampling, respectively. 3D Spiral QTI was implemented as in Gómez et al.8, and was based on a piecewise linear flip angle train of 880 frames, as shown in Figure 1a. 3D Radial QTI flip angle train was numerically optimized by minimizing T1 and T2 Cramer-Rao Lower Bounds9 resulting in the 2000-frames-long schedule shown in Figure 1b. For both variants, the schedule was acquired multiple times (56 and 50 for 3D Spiral and Radial QTI, respectively). The two implementations included an unbalanced gradient along the z axis after each readout to minimize the impact of B0 on signal evolution10, and had constant TR (10 / 6.7 ms for Spiral / Radial) leading to a total scan time of 7 and 12 minutes for 3D Spiral and Radial QTI, respectively, for a 1mm isotropic resolution acquisition with whole-brain coverage. Reconstruction: The reconstruction pipeline consisted of projection of the acquired sampled on a low rank temporal subspace11 followed by interpolation of the acquired sampled to a Cartesian grid12, 3D Fast Fourier Transform, adaptive coil combination13 and orthogonal matching pursuit to a precomputed dictionary of signal evolutions, providing T1 and T2 maps of the tissues. The dictionary was calculated using the Extended Phase Graphs formalism14, and was used to determine the basis of the low rank subspace by SVD decomposition.Validation: To validate the proposed QTI implementations, we scanned 5 gel-filled tubes of a Eurospin TO5 phantom, each with a different T1/T2. Reference values were obtained via gold-standard spin-echo acquisitions (TR=7000ms; TI=[50; 80; 150; 250; 400; 2000; 3000]ms for T1 mapping and TE=[10; 30; 80; 130; 180; 300; 500]ms for T2). Agreement between QTI and reference values was measured both via a correlation plot and by computing Intraclass Correlation Coefficients (ICC). In addition, a healthy volunteer was scanned after obtaining written informed consent, using a matched-resolution MP2RAGE15 acquisition as a reference for T1 and a single slice of a multi-echo spin-echo acquisition for T2, corresponding to the central slice of the QTI acquisitions. The MP2RAGE acquisition was also used as a morphological reference and was segmented to obtain White Matter (WM), Gray Matter (GM) and Cerebrospinal Fluid (CSF) masks. QTI was compared to the reference scans within these three ROIs. All the acquisitions were performed on a GE Signa 7T scanner (GE Healthcare, Waukesha, WI, USA) using a 2-channels Tx / 32-channels Rx head coil (Nova Medical).
Results and Discussion
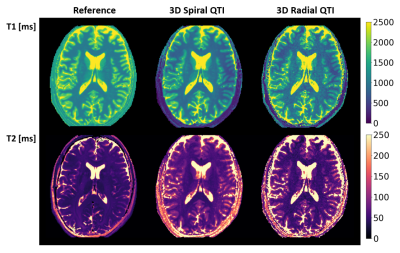
Both 3D Spiral and 3D Radial QTI successfully provided T1 and T2 maps both in-vitro and in-vivo in a reasonable scan time. Reconstruction, which was implemented directly on the MR scanner, was performed in a time compatible with the acquisition time. T1 values for both implementations were in excellent agreement with the reference in-vitro, as shown in Figure 2, resulting in a high ICCs of 0.99. Instead, T2 values obtained using QTI approaches were systematically overestimated compared to reference, resulting in slightly lower ICCs of 0.95 and 0.93 for 3D Spiral QTI and 3D Radial QTI, respectively. This is likely due to the increased transmit field (B1+) inhomogeneity at UHF16. In-vivo, we found good visual agreement with the reference, as reported in Figure 4. In terms of geometrical quality, 3D Radial QTI acquisition showed higher residual undersampling artifacts compared to Spiral but sharper edges due to increased robustness towards B0 effects. Quantitatively, T1 and T2 values were comparable between the two implementations. T1 values were underestimated when compared to reference, likely due to different magnetization transfer (MT) effects between QTI and MP2RAGE17, while T2 were in good agreement with spin-echo measurements in both WM and GM.Conclusion
We successfully obtained T1 and T2 maps of the human brain at 7T in clinically acceptable time. Future works will explore the use of iterative reconstruction algorithms for 3D Radial QTI to reach the acquisition efficiency and undersampling artifact suppression of spiral sampling while maintaining the high anatomical conspicuity of radial trajectories, as well as accounting in the signal model for B1+ and MT effects to correct quantification biases.Acknowledgements
EU FET NICI grant (#801075), EU H2020 CHAIMELEON grant (#952172).
Support from the Italian Ministry of Health via the RC 2022 and “5 per mille” to IRCCS Fondazione Stella Maris.
References
1. Ma D, Gulani V, Seiberlich N, et al. Magnetic resonance fingerprinting. Nature. 2013;495(7440):187-192. doi:10.1038/nature11971
2. Sbrizzi A, Heide O van der, Cloos M, et al. Fast quantitative MRI as a nonlinear tomography problem. Magn Reson Imaging. 2018;46:56-63. doi:10.1016/j.mri.2017.10.015
3. Gómez PA, Molina-Romero M, Buonincontri G, Menzel MI, Menze BH. Designing contrasts for rapid, simultaneous parameter quantification and flow visualization with quantitative transient-state imaging. Sci Rep. 2019;9(1):8468. doi:10.1038/s41598-019-44832-w
4. Jiang Y, Ma D, Keenan KE, Stupic KF, Gulani V, Griswold MA. Repeatability of magnetic resonance fingerprinting T1 and T2 estimates assessed using the ISMRM/NIST MRI system phantom. Magn Reson Med. 2017;78(4):1452-1457. doi:10.1002/mrm.26509
5. Buonincontri G, Biagi L, Retico A, et al. Multi-site repeatability and reproducibility of MR fingerprinting of the healthy brain at 1.5 and 3.0 T. NeuroImage. 2019;195:362-372. doi:10.1016/j.neuroimage.2019.03.047
6. Buonincontri G, Kurzawski JW, Kaggie JD, et al. Three dimensional MRF obtains highly repeatable and reproducible multi-parametric estimations in the healthy human brain at 1.5T and 3T. NeuroImage. 2021;226:117573. doi:10.1016/j.neuroimage.2020.117573
7. Chan RW, Ramsay EA, Cunningham CH, Plewes DB. Temporal stability of adaptive 3D radial MRI using multidimensional golden means. Magn Reson Med. 2009;61(2):354-363. doi:10.1002/mrm.21837
8. Gómez PA, Cencini M, Golbabaee M, et al. Rapid three-dimensional multiparametric MRI with quantitative transient-state imaging. Sci Rep. 2020;10(1):1376
9. doi:10.1038/s41598-020-70789-29. Zhao B, Haldar JP, Liao C, et al. Optimal Experiment Design for Magnetic Resonance Fingerprinting: Cramér-Rao Bound Meets Spin Dynamics. IEEE Trans Med Imaging. 2019;38(3):844-861. doi:10.1109/TMI.2018.2873704
10. Jiang Y, Ma D, Seiberlich N, Gulani V, Griswold MA. MR Fingerprinting Using Fast Imaging with Steady State Precession (FISP) with Spiral Readout. Magn Reson Med. 2015;74(6):1621-1631. doi:10.1002/mrm.25559
11. McGivney DF, Pierre E, Ma D, et al. SVD Compression for Magnetic Resonance Fingerprinting in the Time Domain. IEEE Trans Med Imaging. 2014;33(12):2311-2322. doi:10.1109/TMI.2014.2337321
12. Beatty PJ, Nishimura DG, Pauly JM. Rapid gridding reconstruction with a minimal oversampling ratio. IEEE Trans Med Imaging. 2005;24(6):799-808. doi:10.1109/TMI.2005.848376
13. Walsh DO, Gmitro AF, Marcellin MW. Adaptive reconstruction of phased array MR imagery. Magn Reson Med. 2000;43(5):682-690. doi:https://doi.org/10.1002/(SICI)1522-2594(200005)43:5<682::AID-MRM10>3.0.CO;2-G
14. Weigel M. Extended phase graphs: Dephasing, RF pulses, and echoes - pure and simple. J Magn Reson Imaging. 2015;41(2):266-295. doi:https://doi.org/10.1002/jmri.24619
15. Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele PF, Gruetter R. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. NeuroImage. 2010;49(2):1271-1281. doi:10.1016/j.neuroimage.2009.10.002
16. Buonincontri G, Schulte RF, Cosottini M, Tosetti M. Spiral MR fingerprinting at 7T with simultaneous B1 estimation. Magn Reson Imaging. 2017;41:1-6. doi:10.1016/j.mri.2017.04.003
17. A.G. Teixeira RP, Malik SJ, Hajnal JV. Fast quantitative MRI using controlled saturation magnetization transfer. Magn Reson Med. 2019;81(2):907-920. doi:10.1002/mrm.27442
Figures