1361
A Rapid, Whole-brain Quantification of T1 Relaxation Time Using Submillimetre Inversion-recovery EPI at 7T
Seong Dae Yun1 and N. Jon Shah1,2,3,4
1Institute of Neuroscience and Medicine 4, INM-4, Forschungszentrum Juelich, Juelich, Germany, 2Institute of Neuroscience and Medicine 11, INM-11, JARA, Forschungszentrum Juelich, Juelich, Germany, 3JARA - BRAIN - Translational Medicine, Aachen, Germany, 4Department of Neurology, RWTH Aachen University, Aachen, Germany
1Institute of Neuroscience and Medicine 4, INM-4, Forschungszentrum Juelich, Juelich, Germany, 2Institute of Neuroscience and Medicine 11, INM-11, JARA, Forschungszentrum Juelich, Juelich, Germany, 3JARA - BRAIN - Translational Medicine, Aachen, Germany, 4Department of Neurology, RWTH Aachen University, Aachen, Germany
Synopsis
Keywords: Contrast Mechanisms, Quantitative Imaging, 7T, Inversion-recovery EPI, Rapid T1 mapping, Submillimetre and Whole-brain
Knowledge of T1 relaxation time is of great interest for clinical diagnosis or MRI sequence optimisation. For the quantitative measurement of T1, the inversion-recovery method is widely used due to its relatively good accuracy or tolerance to B1 inhomogeneity. However, 2D multi-slice- or 3D segmentation-based readout methods often preclude the effect of different signal recovery modulation depending on slice locations. Therefore, this work presents a single-slice-based inversion-recovery 2D EPI method, combined with TR-external EPI phase correction at 7T, to provide rapid, whole-brain T1 mapping with a voxel size of 0.73 × 0.73 mm2.Introduction
The quantitative measurement of T1 relaxation time is of great interest in the MR community as it can provide valuable information on physiological state for clinical diagnosis or physical tissue parameters to optimise imaging sequences.1-4 Previously, various methods have been presented for T1 mapping, and of these, the inversion-recovery method is considered a gold standard method, as it can provide an enhanced accuracy or tolerance to B1 inhomogeneity when compared to other types of methods (e.g. variable flip angle).1,5 However, the approach of tracking the dynamic recovery of longitudinal magnetisation after an inversion inherently requires a relatively long acquisition time, which can significantly hinder the mapping of whole-brain T1 with high spatial resolution. To effectively shorten its acquisition time, an inversion-recovery method, the readout of which is implemented with echo-planar-imaging, namely IR-EPI, was demonstrated using the 2D multi-slice-based or 3D segmented EPI regime.1,5,6 However, the acquisition of multiple slices in successive excitation loops within the same T1 recovery period can impose a different signal recovery modulation for each slice. That is, the earlier time point during the recovery may not be effectively captured in the slice acquired at a later time point. Specifically, this problem becomes more severe as the number of slices increases for larger brain coverage. Therefore, this work presents a single-slice-based IR-EPI method (i.e. one inversion-recovery per each slice) where the number of required inversion-recovery loops is reduced by means of the multiband acceleration, resulting in a much shorter acquisition time.7,8 Moreover, the EPI readout was further configured with the TR-external EPI phase correction scheme (TRx) to substantially reduce the minimum TE required for submillimetre resolution EPI.9,10Methods
Figure 1 shows a diagram of the proposed imaging sequence. The inversion-recovery loop is repeated as many times as the number of slices, which can be reduced by the multiband acceleration. Each inversion-recovery kernel starts with combined saturation and inversion preparation pulses and subsequently performs dynamic sampling only for a single slice or a group of slices in case of multiband acceleration. This strategy ensures data at each slice location have identical inversion time points. Here, each temporal point is acquired with 2D EPI combined with the TRx scheme. The feasibility of using the multiband and the TRx techniques for IR-EPI was verified by comparing the signals from the following four sequences: 1) IR-EPI, 2) IR-Multiband EPI (IR-MEPI), 3) TRx-IR-EPI and 4) TRx-IR-MEPI. For this comparison, low-resolution data sets from a uniform spherical phantom were acquired without any acceleration condition (see Fig. 2a). Next, submillimetre-resolution protocols were configured using IR-MEPI and TRx-IR-MEPI, each of which was optimised to offer the highest possible spatial resolutions (0.97 × 0.97 mm2 and 0.73 × 0.73 mm2, respectively) under the imaging condition shown in Fig. 2b. Data sets from a healthy volunteer screened with a standard safety procedure were acquired using the two submillimetre protocols. All experiments in this work were performed at a Siemens Magnetom Terra 7T scanner with a 1-Tx/32-Rx head coil.Results
Figures 3a-c depict the temporal signal evolution of the phantom data obtained from the four different sequences. This result suggests that the use of the multiband or TRx techniques has no significant impact on the behaviour of the signal recovery. The very small difference observed in the above plots was also numerically verified by computing the mean signal differences (see Fig. 3d). Figure 4 shows T1 maps at a representative slice location obtained from IR-MEPI (0.97 × 0.97 mm2) and TRx-IR-MEPI (0.73 × 0.73 mm2). This result demonstrates the impact of the superior spatial resolution achieved in TRx-IR-MEPI, resulting in a clearer delineation of the T1 values around the cortical ribbon. Figure 5 shows the T1 maps for all the slices (75) from TRx-IR-MEPI, and depicts reliable T1 estimation for all slice locations. The mean T1 values for whole-brain WM/GM were computed as 1200.72/1640.30 ms for IR-MEPI and 1140.20/1617.19 ms for TRx-IR-MEPI, both of which are in good agreement with those reported in previous submillimetre T1 mapping studies obtained with MP2RAGE (1140/1653)5, IR-Turbo-spin-echo (1017/1654)11 or IR-EPI (1170/1783)5 methods at 7T.Discussion and conclusions
This work demonstrates rapid, whole-brain T1 mapping using TRx-IR-MEPI at 7T. The method was able to achieve a relatively large matrix size, consequently resulting in a substantial improvement in the spatial resolution of T1 maps when compared to IR-MEPI. The achieved spatial resolution suggests its potential use for cortical depth-dependent T1 quantification. The TE employed here was 17.6 ms, which was also similar to the TEs (16~20 ms) employed in the previous submillimetre IR-EPI study.5 However, in contrast, our method is based on single-shot EPI, which can mitigate increased aliasing artefacts, physiological noise or subject motion stemming from the multiple shots typically seen in 2D multi-shot or 3D segmented EPI. For the submillimetre-protocols tested here, the required time for the entire T1 recovery data (0.73 × 0.73 mm2 × 75 slices × 60 time-points) was only 3.5 minutes, which was mainly the result of the use of the multiband acceleration. In future works, a study with more subjects including the inversion efficiency measurement12,13 is planned for more quantitative analysis on TRx-IR-MEPI with improved T1 mapping accuracy.Acknowledgements
No acknowledgement found.References
- Wright PJ, Mougin OE, Totman JJ, et al. Water proton T1 measurements in brain tissue at 7, 3, and 1.5 T using IR-EPI, IR-TSE, and MPRAGE: results and optimization. MAGMA. 2008;21(1-2):121-130.
- Paus T, CollinsD, EvansAet al (2001) Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull 54:255–266.
- Makhijani MK, Hu HH, Pohost GM, Nayak KS. Improved blood suppression in three-dimensional (3D) fast spin-echo (FSE) vessel wall imaging using a combination of double inversion-recovery (DIR) and diffusion sensitizing gradient (DSG) preparations. J Magn Reson Imaging. 2010 Feb;31(2):398-405. doi: 10.1002/jmri.22042. PMID: 20099353; PMCID: PMC6570529.
- Del Grande F, Santini F, Herzka DA, et al. Fat-suppression techniques for 3-T MR imaging of the musculoskeletal system. RadioGraphics 2014; 34:217-233.
- Sanchez Panchuelo RM, Mougin O, Turner R, Francis ST. Quantitative T1 mapping using multi-slice multi-shot inversion-recovery EPI. Neuroimage. 2021 Jul 1;234:117976. doi: 10.1016/j.neuroimage.2021.117976. Epub 2021 Mar 26. PMID: 33781969; PMCID: PMC8204273.
- Stirnberg R, Dong Y, Bause J, Ehses P, Stöcker T. T1 Mapping at 7T Using a Novel Inversion-Recovery Look-Locker 3D-EPI Sequence. In Proceedings of the 27th Annual Meeting of ISMRM, Montreal, Canada, 2019. Abstract 4420.
- Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Gunther M, et al. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PLoS One. 2010;5(12):e15710.
- Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med. 2012 May;67(5):1210-24.
- Yun SD, Shah NJ. Analysis of EPI phase correction with low flip-angle excitation to reduce the required minimum TE: Application to whole-brain, submillimeter-resolution FMRI at 3 T. Magn Reson Med. 2020; 84: 1416– 1429. https://doi.org/10.1002/mrm.28218.
- Yun SD, Pais-Roldán P, Palomero-Gallagher N, Shah NJ. Mapping of whole-cerebrum resting-state networks using ultra-high resolution acquisition protocols. Human Brain Mapping. 2022;43(11):3386-3403. https://doi.org/10.1002/hbm.25855.
- Wright PJ, Peters A. Brookes M, Coxon R, Morris P, Francis S, Bowtell R, Gowland P. T1 Measurements for Cortical Grey Matter, White Matter and Sub-Cortical Grey Matter at 7T. In Proceedings of the 14th Annual Meeting of ISMRM, Seattle, USA, 2006. p 921.
- Raman, F.S., Kawel-Boehm, N., Gai, N. et al. Modified look-locker inversion recovery T1 mapping indices: assessment of accuracy and reproducibility between magnetic resonance scanners. J Cardiovasc Magn Reson 15, 64 (2013). https://doi.org/10.1186/1532-429X-15-64.
- Kellman, P., Herzka, D.A. and Hansen, M.S. (2014), Adiabatic inversion pulses for myocardial T1 mapping. Magn. Reson. Med., 71: 1428-1434. https://doi.org/10.1002/mrm.24793
Figures
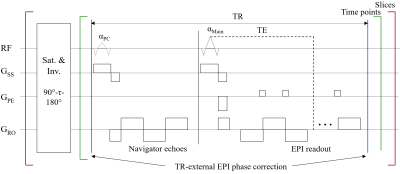
Figure 1. Sequence diagram of proposed
IR-EPI with TR-external EPI phase correction (TRx-IR-EPI). The inversion-recovery loop is repeated as
many times as the number of slices (i.e. the bracket marked in red) and can be
reduced by a factor of the multiband acceleration rate. The saturation and
inversion preparation pulses are the adiabatic, non-selective 90°-τ-180° magnetisation
preparation (tau = 2.4 s). Each temporal point is sampled with TRx-EPI where
the navigator echoes are acquired in a separate loop to avoid the increase of
the minimum TE required (αPC = 9°).
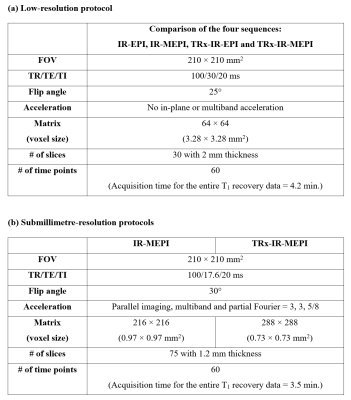
Figure 2. (a) A low-resolution (voxel: 3.28 × 3.28 mm2) protocol to check the feasibility
of using the multiband technique and the TRx scheme for IR-EPI. (b) Submillimetre-resolution protocols
configured with IR-MEPI (voxel: 0.97 × 0.97 mm2) and TRx-IR-MEPI (voxel: 0.73 × 0.73
mm2) where
each protocol was optimised to offer its highest possible spatial resolution under
the imaging conditions shown in the table.
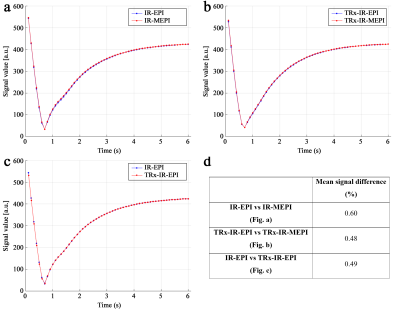
Figure 3. Comparison of the temporal signal
evolution from the four different inversion-recovery sequences for the
following pairs: (a) IR-EPI and
IR-MEPI, (b) TRx-IR-EPI and
TRx-IR-MEPI and (c) IR-EPI and
TRx-IR-EPI. The signal curve was obtained by taking the mean value of the
phantom data at all slice locations. (d)
The mean signal difference of the signal curves is plotted in the image panels
from a to c. The results shown here suggest the feasibility of the multiband
technique and TRx scheme in the inversion-recovery EPI method.
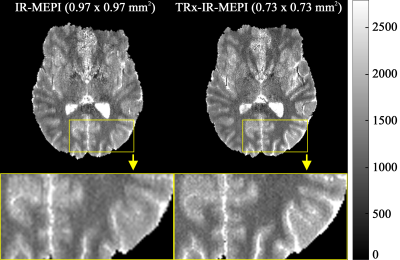
Figure 4. Submillimetre T1 maps
at a representative slice location obtained from IR-MEPI and TRx-IR-MEPI. The enlarged
depiction of the selected ROI (see below each slice) demonstrates the use of a
superior spatial resolution in TRx-IR-MEPI (0.73 × 0.73 mm2) in comparison to
IR-MEPI (0.97 × 0.97
mm2). A clearer delineation of T1 values around the
cortical ribbon can be observed in TRx-IR-MEPI.
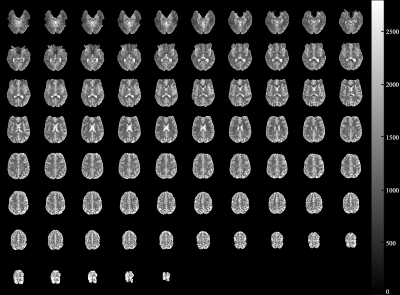
Figure 5. Whole-brain T1 maps (0.73
× 0.73 mm2
× 75 slices) obtained from TRx-IR-MEPI. The acquisition time required for the T1
recovery data with 60 time points was 3.5 minutes.
DOI: https://doi.org/10.58530/2023/1361