1347
Dose finding and sequence tailoring in sentinel lymph node detection of tongue cancer using superparagmagnetic iron-oxide particles1Department of Otorhinolaryngology and Head and Neck Surgery, Radboud University Medical Center, Nijmegen, Netherlands, 2Department of Radiation Oncology, Radboud University Medical Center, Nijmegen, Netherlands, 3Department of Medical Imaging, Radboud University Medical Center, Nijmegen, Netherlands, 4Department of Oral- and Maxillofacial Surgery and Head and Neck Surgery, Radboud University Medical Center, Nijmegen, Netherlands, 5Department of Pathology, Radboud University Medical Center, Nijmegen, Netherlands
Synopsis
Keywords: Cancer, Head & Neck/ENT
To clarify the amount, timing and optimal pulse sequence settings of using interstitial superparamagnetic iron oxide particles (SPIO) as a contrast medium to detect sentinel lymph nodes in head and neck radiology, we injected SPIO peritumorally in six patients with early-stage oral squamous cell carcinoma. Anatomical and T2*-weighted MR images were acquired, and dose was altered after every two patients. Images with different computed echo times were created to determine the signal attenuation effect of SPIO on the sentinel lymph nodes.Introduction
In early-stage oral squamous cell carcinoma (OSCC) with a clinically negative neck, sentinel lymph node biopsy (SLNB) is currently standard-of-care1. It comprises peritumoral injection with radioactively labelled tracer, localization of sentinel lymph nodes (SLNs) on lymphoscintigraphy and single photon emission computed tomography (SPECT) and CT, and resection using a gamma probe intraoperatively. It is an invasive method that lacks sensitivity when used in floor-of-mouth tumors due to the shine-through phenomenon: SLNs close to the primary tumor can go undetected because of the high tracer activity of injection sites2.Superparamagnetic iron-oxide particles3 (SPIO) have been investigated as a tracer to detect SLNs with MRI and seem a promising possibility due to high spatial resolution of MRI compared to SPECT. SPIO were initially intended for use with a magnetic probe in oncologic surgery4, which requires an injection dose of 0.4ml of SPIO to detect SLNs intraoperatively5. SPIO can also be used as an MRI contrast medium6. If feasible and accurate, can be used to develop a radiological, non-invasive staging method of the neck as there is evidence that SPIO is only taken up in non-metastatic (parts of) lymph nodes, and not by metastatic foci.4, 6 This way, nodal stage could be determined based on MRI. With insufficient amount of SPIO, SLNs could go undetected, whereas excessive amount of SPIO causes a distorting level of susceptibility artifacts, known as “blooming”. This study was done to clarify the amount, timing and optimal pulse sequence settings of using SPIO as a contrast medium in head and neck radiology.
Methods
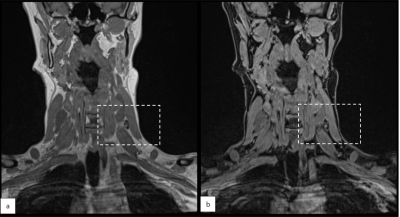
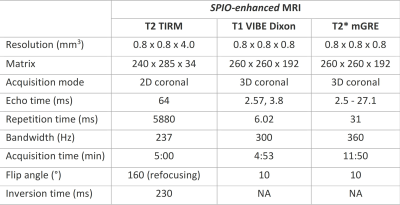
In six patients with early-stage OSCC eligible for SLNB, Magtrace (Endomagnetics Ltd, Cambridge, UK) was injected submucosally into four peritumoral injection sites around the primary tumor, after which an MRI was acquired within 1 hour. Images were evaluated after every two patients to determine the next step in dose-finding. If there was little signal attenuation, the dose was elevated. The dose was lowered if there was too much signal attenuation to delineate the lymph node and recognize surrounding structures.MRI-examinations were carried out on a 3 T MR system with a head-neck coil (Siemens Magnetom Prisma, Erlangen, Germany). Protocol consisted of coronal turbo inversion recovery magnitude (TIRM) T2-weighted images, 3-dimensional (D) Dixon-volume interpolated breath-hold examination (VIBE) T1-weighted images, and 3D multi gradient echo (mGRE) T2*-weighted images (Table 2). Dixon imaging provided water, lipid, in- and opposed phase image sets, and mGRE T2*-weighted images were used to calculate computed echo time images (cTE)7. Isotropic resolution of 0.8mm was used. When patient movement distorted the image, mGRE was repeated at lower spatial resolution (1.2mm isotropic).
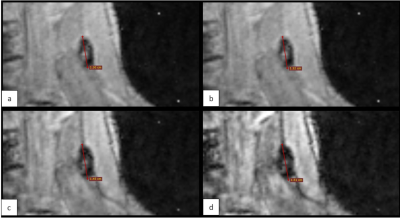
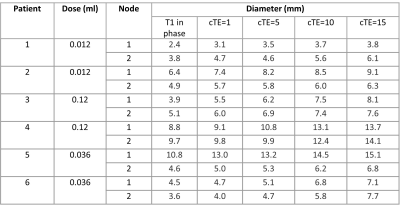
To measure the effect of cTE on signal attenuation and blooming due to SPIO, the largest diameter of the SLNs was measured on coronal water and in-phase images from the Dixon sequence, and compared to the diameters of the signal attenuation at the location of the SLNs with different cTEs.
Results
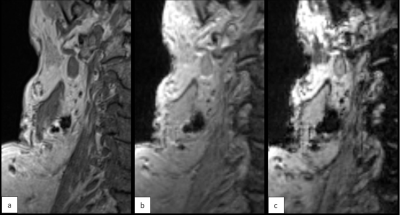
Consecutive doses per two patients were 0.012ml, 0.12ml and 0.036ml. Alterations were done after patient 1&2 because signal attenuation was marginal, and after patient 3&4 because there was too much blooming. At a dose of 0.036ml, the area of signal attenuation can become larger than 1.5 times the size of the lymph node as measured on the anatomical image (Table 1). At cTEs of 10 and 15ms, this can cause overlapping signal attenuation of lymph nodes, making it difficult to discern and delineate them (Figure 3). A cTE of 5ms created the most detailed image with SPIO still detectable. After six included patients comprising two alterations in dose we show that 0.036ml Magtrace (1.004 mg iron) contributed to the most detailed images.Discussion
This study shows using interstitial SPIO is feasible for detecting SLNs on MRI. The dose of SPIO is primarily based on use combined with an intraoperative magnetometer. Magtrace and its precursor Sienna+ are advised to use in a dose of 1-2ml (27.5-55mg iron), whereas these results show a much lower dose of 0.036ml is most suited in using interstitial SPIO as an MRI contrast agent at a magnetic field strength of 3T. The necessary SPIO dose for these two independent approaches to finding the SLNs is very different.In T2*-weighted imaging of these SPIOs, cTEs can be used to fine-tune the amount of blooming and thus the accurate imaging of the SLNs. In this study, we calculated cTEs of 1, 5, 10 and 15ms. In future research, a radiologist should be involved in assessing general image quality and nodal detection at longer cTE determining which cTE is most suited, or more quantitative susceptibility measures could be assessed.
In the upcoming four patients that will be included, dose wil not be altered as we reached sufficient detail and SPIO detection. Future research will also investigate differences in uptake intranodally and if this correlates with presence of metastatic cells on histopathology. SLNs on MRI will be matched with SPECT-CT and pathology to evaluate concordance.
Conclusion
The results of this study will help set up adequate MRI sequences at 3T to detect SLNs in head and neck cancer and can contribute to the development of a non-invasive method to stage the neck in early-stage OSCC patients.Acknowledgements
This research was funded by the Dutch Cancer Society (KWF 2021-2 / 14109).References
1. Schilling C, Stoeckli SJ, Vigili MG, de Bree R, Lai SY, Alvarez J, et al. Surgical consensus guidelines on sentinel node biopsy (SNB) in patients with oral cancer. Head Neck. 2019;41(8):2655-64.
2. de Bree R, de Keizer B, Civantos FJ, Takes RP, Rodrigo JP, Hernandez-Prera JC, et al. What is the role of sentinel lymph node biopsy in the management of oral cancer in 2020? Eur Arch Otorhinolaryngol. 2021;278(9):3181-91.
3. Wang YX, Idee JM. A comprehensive literatures update of clinical researches of superparamagnetic resonance iron oxide nanoparticles for magnetic resonance imaging. Quant Imaging Med Surg. 2017;7(1):88-122.
4. Motomura K, Ishitobi M, Komoike Y, Koyama H, Noguchi A, Sumino H, et al. SPIO-enhanced magnetic resonance imaging for the detection of metastases in sentinel nodes localized by computed tomography lymphography in patients with breast cancer. Ann Surg Oncol. 2011;18(12):3422-9.
5. Nieuwenhuis ER, Kolenaar B, van Bemmel AJM, Hof JJ, van Baarlen J, Christenhusz A, et al. A complete magnetic sentinel lymph node biopsy procedure in oral cancer patients: A pilot study. Oral Oncol. 2021;121:105464.
6. Motomura K, Tabuchi Y, Enomoto Y, Nishida T, Nakaoka T, Mori D, et al. Accurate axillary staging by superparamagnetic iron oxide-enhanced MRI at 1.5 T with fat-suppression sequence as an alternative to sentinel node biopsy in breast cancer. Br J Surg. 2021;108(11):e359-e60.
7. Philips BWJ, Stijns RCH, Rietsch SHG, Brunheim S, Barentsz JO, Fortuin AS, et al. USPIO-enhanced MRI of pelvic lymph nodes at 7-T: preliminary experience. Eur Radiol. 2019;29(12):6529-38.
Figures