1343
Disrupted within-network segregations and between-network integrations in age-related hearing loss with cognitive decline1Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China, 2Nanjing First Hospital, Nanjing Medical University, Nanjing, China, 3University at Buffalo, The State University of New York, Buffalo, NY, United States
Synopsis
Keywords: Head & Neck/ENT, fMRI (resting state), Brain Connectivity, Degenerative, Dementia, Neuroscience
Age-related hearing loss is generally associated with dementia. However, the underlying mechanisms and causal relationship linking ARHL to dementia are poorly understood. Based on resting-state fMRI, the study found that ARHL disrupts specific aspects of resting-state functional connectivity patterns across frontal-parietal regions of the central nervous system; these changes presumably reflect cortical reorganization resulting from auditory sensory deprivation and/or the long-term consequences of effortful listening. The ARHL disruption of network information processing presumably accelerates brain aging and contributes to cognitive decline.Background and Objectives
Age-related hearing loss (ARHL), one of the most common neurodegenerative disorders, is considered the largest modifiable risk factor for dementia in older adults1,2. How ARHL contributes to the development of dementia is poorly understood, but the loss of sensory information increases listening effort and social isolation, factors that likely disrupt neural networks involved in memory, cognition and executive function3-6. The cross-sectional observational study was designed to characterize the cognitive related functional network reorganization induced by ARHL.Methods
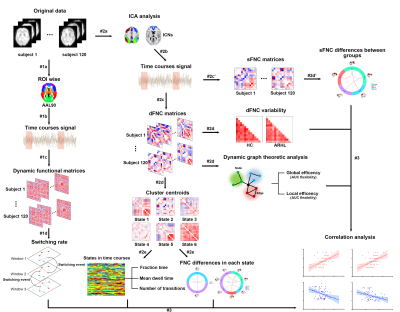
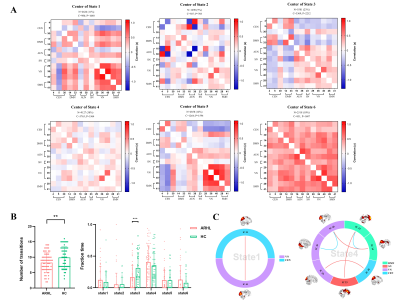
Resting-state functional MRI and cognitive functions were assessed in 66 ARHL patients and 54 healthy controls. Group spatial independent component analyses, sliding window analyses, graph-theory methods and multilayer networks were used to identify ARHL-induced disturbances in static and dynamic functional network connectivity (sFNC/dFNC) and alterations in global network switching. Two-sample t-tests and Mann-Whitney U-tests were used for group-level differences of each feature.Results
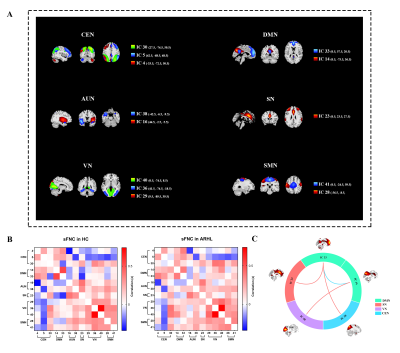
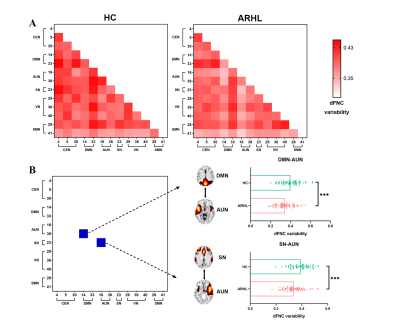
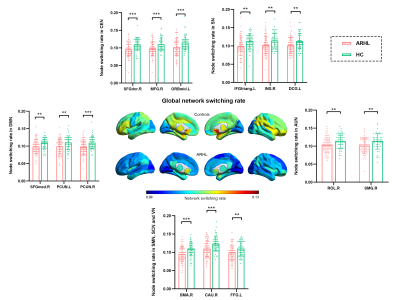
ARHL was associated with decreased sFNC and dFNC within the default mode network (DMN), increased sFNC and dFNC between the DMN and the central executive network (CEN), salience network (SN) and visual network (VN). ARHL was associated with decreased variability in dFNC between the DMN and the auditory network (AUN) and between the salience network (SN) and AUN. Patients with ARHL had lower network switching rates than controls among global network nodes, especially in the DMN.Conclusion
The prolonged loss of sensory information, social isolation and increased listening effort associated with ARHL induced compensatory within-network segregations and between-network integrations in the DMN, and reduced network information processing, disturbances that could accelerate cognitive decline and brain aging.Acknowledgements
The authors thank all the study participants for assistance with recruitment and data collection.References
1.Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of Age-Related Hearing Loss With Cognitive Function, Cognitive Impairment, and Dementia: A Systematic Review and Meta-analysis. JAMA Otolaryngol Head Neck Surg 2018; 144(2): 115-126.
2.Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of Age-Related Hearing Loss With Cognitive Function, Cognitive Impairment, and Dementia: A Systematic Review and Meta-analysis. JAMA Otolaryngol Head Neck Surg 2018; 144(2): 115-126.
3.Jafari Z, Kolb BE, Mohajerani MH. Age-related hearing loss and tinnitus, dementia risk, and auditory amplification outcomes. Ageing Res Rev 2019; 56: 100963.
4.Slade K, Plack CJ, Nuttall HE. The Effects of Age-Related Hearing Loss on the Brain and Cognitive Function. Trends in neurosciences 2020; 43(10): 810-821.
5.Rosemann S, Thiel CM. Audio-visual speech processing in age-related hearing loss: Stronger integration and increased frontal lobe recruitment. Neuroimage 2018; 175: 425-437.
6.Johnson JCS, Marshall CR, Weil RS, Bamiou DE, Hardy CJD, Warren JD. Hearing and dementia: from ears to brain. Brain 2021; 144(2): 391-401.
Figures