1331
Use of diffusion kurtosis imaging and dynamic contrast-enhanced MRI in differentiating parotid gland tumors
zijun Liu1, baohong Wen1, and yan Zhang1
1The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
1The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Synopsis
Keywords: Head & Neck/ENT, Head & Neck/ENT, Parotid gland tumors
This study evaluates the usefulness of combined Diffusion kurtosis imaging (DKI) and dynamic contrast-enhanced MRI (DCE-MRI) in differentiating parotid gland tumors. DKI and DCE-MRI quantitative parameters were analyzed by using the Kruskal-Wallis H test and post hoc test with bonferroni correction or the one-way analysis of variance (ANOVA) with LSD method, and ROC curve. Our research shows that the significant parameters in stepwise diagnosing parotid gland tumors were Kep, Ktrans, and D value. Therefore, the combined use of DKI and DCE-MRI could be used to differentiate various parotid gland tumors, and it may be helpful for differentiating parotid gland tumors.Introduction
Parotid gland tumors (PGTs) contain rich histological types and subtypes, and most of them are benign, including pleomorphic adenomas (PAs), Warthin tumors (WTs), and basal cell adenomas (BCAs). Although the malignant tumors (MTs) have the lower incidence, cases have been increasing. The treatment strategies and prognoses are usually very different because of the various histological types of these tumors1. Therefore, accurate preoperative diagnosis takes an important part. DKI can provide two parameters, diffusion kurtosis (K) and diffusion coefficient (D), by quantifying the non-Gaussian behavior of water molecule diffusion. DCE-MRI can convey hemodynamic information, and provide quantitative parameters to distinguish benign and malignant lesions. Previous studies showed that DKI was useful in examining parotid gland lesions2,3. And quantitative DCE-MRI has taken an important role in diagnosing parotid gland lesions4-6. However, few previous studies demonstrated the added value of combining DKI and DCE-MRI for parotid gland tumors characterization. Therefore, the aim of this study is to evaluate the diagnostic performance of combined diffusion kurtosis imaging (DKI) and dynamic contrast-enhanced MRI (DCE-MRI) in the differentiating parotid gland tumors.Methods
One hundred and forty-two patients with histopathologically confirmed parotid gland tumors were enrolled and divided into four groups: pleomorphic adenomas (PAs)(n=68), Warthin tumors (WTs)(n=19), basal cell adenomas (BCAs)(n=11), and malignant tumors (MTs)(n=44). All patients underwent MRI on a 3.0-T scanner (Skyra; Siemens Healthcare) with a 20-channel head/neck coil to get DKI and DCE-MRI quantitative parameters. Using Kolmogorov-Smirnov’s test and Levene’s test to test all numeric data for their normality and homogeneity of variance. Using the Kruskal-Wallis H test, post hoc test with Bonferroni correction or the one-way analysis of variance (ANOVA), and post hoc test with LSD method, and ROC curve to analyze DKI and DCE-MRI quantitative parameters. Comparisons of AUCs were analyzed by Delong test. The intraclass correlation coefficient (ICC) with 95% confidence intervals (CIs) was calculated to evaluate the agreement of the two physicians’ imaging interpretations.Results
The D value and the K value were the significant parameters in distinguishing the BTs from the MTs. The Kruskal–Wallis H test and the one-way analysis of variance (ANOVA) revealed that there were statistically significant differences in all quantitative parameters among four groups of PGTs (all p<0.05), and the comparisons of quantitative parameters of PGTs are summarized in Table 1. PAs demonstrated the lowest Ktrans value, Kep value, and iAUC value compared with WTs, BCAs, MTs (all p<0.05). WTs had the lower D value compared with BCAs and PAs (all p<0.001), and MTs had the lower Kep value compared with WTs and BCAs (all p<0.05). The diagnostic performances between the different groups were summarized in Table 2. For DKI parameters, as exhibited in Figure 1. Optimal cutoff values and diagnostic performance of DKI and DCE-MRI parameters for stepwise discrimination were shown in Table 3 and Figure 2. ROC analyses showed that in differentiating PAs from other groups, the combination of Kep and Ktrans values (AUC, 0.86) generated better diagnostic performance than Ktrans (P<0.001), but the difference in AUC between the combination of Kep and Ktrans values and Kep did not reach significance (p=0.3). The Kep value (AUC, 0.84) was useful for differentiating MTs from WTs and BCAs. And the D value (AUC, 0.86) was used to differentiate WTs from BCAs. Excellent inter- and intraobserver consistency for DKI and DCE-MRI parameters was achieved with ICCs ranging from 0.84 to 0.98.Discussion
Our study showed that the combination of DKI and DCE-MRI can be good at differentiating PGTs. In our study, with a relatively big sample size, The D value of BTs was significantly higher than that of MTs, and the K value of BTs was significantly lower than that of MTs, which was consistent with the previous study7. However, the K value of MTs was not significantly different from that of WTs and BCAs, perhaps the sample size of PAs was so big that the K value of BTs was influenced. WTs had the higher Kep value than PAs and MTs, and had the lower Ve value than PAs, but the Ve value between WTs and MTs showed insignificant differences, which was incompatible with other studies8. However, DCE-MRI parameters did not distinguish WTs from BCAs. The reason for this phenomenon perhaps was the limited extracellular and extravascular space in WTs 5, and a great number of vascular structures in BCAs9. But the D value was useful to differ WTs from BCAs. In the future, studies with a bigger sample size are needed to explore the differences of quantitative parameters between MTs and BCAs. This study demonstrated that DKI or DCE-MRI could not differentiate PGTs respectively, but the combination of DKI and DCE-MRI could be used to differentiate PGTs.Conclusions
In this study, we aimed at using the combination of DKI and DCE-MRI for differentiating various parotid gland tumors, which showed very good performance. Further studies with the combination of DKI and DCE-MRI are mandatory to distinguish PGTs.Acknowledgements
No acknowledgement found.References
- Gatta G, Guzzo M, Locati LD, McGurk M, Prott FJ. Major and minor salivary gland tumours. Crit Rev Oncol Hematol. 2020 Aug;152:102959. doi: 10.1016/j.critrevonc.2020.102959. Epub 2020 May 18. PMID: 32485526.
- Yu S, Zhang Z, Bao Q, Su J, Liu M, Shi Q, Cai W. Diffusion kurtosis imaging in the differential diagnosis of parotid gland disease and parotid adenolymphoma: preliminary results. Dentomaxillofac Radiol. 2018 Jul;47(6):20170388. doi: 10.1259/dmfr.20170388. Epub 2018 May 16. PMID: 29676939; PMCID: PMC6196054.
- Qian W, Xu XQ, Zhu LN, Ma G, Su GY, Bu SS, Wu FY. Preliminary study of using diffusion kurtosis imaging for characterizing parotid gland tumors. Acta Radiol. 2019 Jul;60(7):887-894. doi: 10.1177/0284185118803784. Epub 2018 Sep 27. PMID: 30259752.
- Yabuuchi H, Kamitani T, Sagiyama K, Yamasaki Y, Hida T, Matsuura Y, Hino T, Murayama Y, Yasumatsu R, Yamamoto H. Characterization of parotid gland tumors: added value of permeability MR imaging to DWI and DCE-MRI. Eur Radiol. 2020 Dec;30(12):6402-6412. doi: 10.1007/s00330-020-07004-3. Epub 2020 Jul 1. PMID: 32613285.
- Xu Z, Zheng S, Pan A, Cheng X, Gao M. A multiparametric analysis based on DCE-MRI to improve the accuracy of parotid tumor discrimination. Eur J Nucl Med Mol Imaging. 2019 Oct;46(11):2228-2234. doi: 10.1007/s00259-019-04447-9. Epub 2019 Aug 1. Erratum in: Eur J Nucl Med Mol Imaging. 2020 Apr;47(4):1017. PMID: 31372671.
- Patella F, Franceschelli G, Petrillo M, Sansone M, Fusco R, Pesapane F, Pompili G, Ierardi AM, Saibene AM, Moneghini L, Biglioli F, Carrafiello G. A multiparametric analysis combining DCE-MRI- and IVIM -derived parameters to improve differentiation of parotid tumors: a pilot study. Future Oncol. 2018 Dec;14(28):2893-2903. doi: 10.2217/fon-2017-0655. Epub 2018 Feb 9. PMID: 29425058.
- Ma G, Xu XQ, Hu H, Su GY, Shen J, Shi HB, Wu FY. Utility of Readout-Segmented Echo-Planar Imaging-Based Diffusion Kurtosis Imaging for Differentiating Malignant from Benign Masses in Head and Neck Region. Korean J Radiol. 2018 May-Jun;19(3):443-451. doi: 10.3348/kjr.2018.19.3.443. Epub 2018 Apr 6. PMID: 29713222; PMCID: PMC5904471.
- Huang N, Chen Y, She D, Xing Z, Chen T, Cao D. Diffusion kurtosis imaging and dynamic contrast-enhanced MRI for the differentiation of parotid gland tumors. Eur Radiol. 2022 Apr;32(4):2748-2759. doi: 10.1007/s00330-021-08312-y. Epub 2021 Oct 12. PMID: 34642805; PMCID: PMC8921043.
- Shi L, Wang YX, Yu C, Zhao F, Kuang PD, Shao GL. CT and ultrasound features of basal cell adenoma of the parotid gland: a report of 22 cases with pathologic correlation. AJNR Am J Neuroradiol. 2012 Mar;33(3):434-8. doi: 10.3174/ajnr.A2807. Epub 2011 Dec 22. PMID: 22194377; PMCID: PMC7966456.
Figures
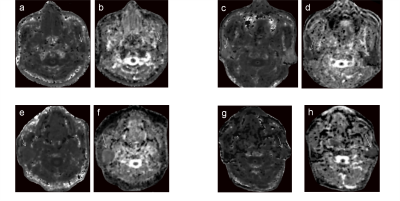
Figure 1: a–b: A 31-year-old woman with pleomorphic adenoma. DKI parameter maps showed the K (a), D (b) values of 0.62 and 1.55×10-3mm2/s, respectively. c-d: A 47-year-old man with Warthin tumor. DKI parameter maps showed the K (c), D (d) values of 1.63 and 0.89×10-3mm2/s, respectively. e-f: A 72-year-old woman with basal cell adenoma. DKI parameter maps showed the K (e), D (f) values of 0.68 and 1.41×10-3mm2/s, respectively. g-h: A 63-year-old man with salivary duct carcinoma. DKI parameter maps showed the K (g), D (h) values of 0.67 and 1.36×10-3mm2/s, respectively.
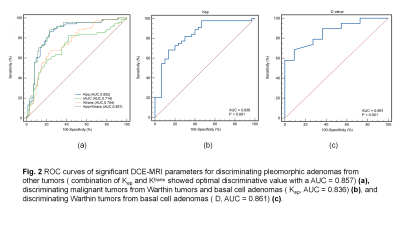
Figure 2: Optimal diagnostic performance of DKI and DCE-MRI parameters for stepwise discrimination
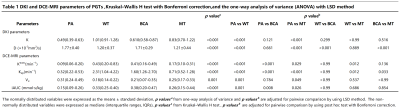
Table 1: DKI and DCE-MRI parameters of PGTs ,Kruskal–Wallis H test with Bonferroni correction,and the one-way analysis of variance (ANOVA) with LSD method
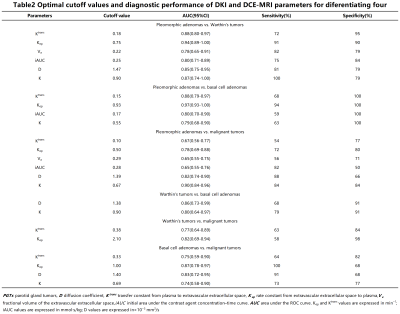
Table 2: Optimal cutoff values and diagnostic performance of DKI and DCE-MRI parameters for diferentiating four groups of PGTs

Table 3: Optimal cutoff values and diagnostic performance of DKI and DCE-MRI parameters for stepwise discrimination
DOI: https://doi.org/10.58530/2023/1331